A research team from Donghua University (DHU) has achieved a major breakthrough in Organic Solvent Nanofiltration (OSN), developing a new generation of high-performance polyamide membranes capable of ultrafast filtration in both polar and non-polar solvents. The findings, led by Prof. Wu Peiyi and Prof. Wu Huiqing of the College of Chemistry and Chemical Engineering, were recently published in Nature Communications under the title “Microstructure engineering of polyamide membranes for ultrafast polar and non-polar solvent transport” (Nat. Commun. 2025, 16, 8414).
OSN has emerged as a critical technology for the chemical, pharmaceutical, and petrochemical industries, enabling molecule-level separation and purification in organic media. However, as industrial solvent systems become more diverse and chemically complex, industry demand has outpaced the capabilities of traditional nanofiltration membranes.
Polyamide thin-film composite (TFC) membranes—currently the industry benchmark—often struggle with excessive thickness, dense structures, and strong hydrophilicity. These characteristics limit permeability in polar solvents and result in particularly poor performance with non-polar solvents. Much of this stems from limitations inherent to conventional water/alkane interfacial polymerization (IP), which restricts the available monomer types and yields heterogeneous, thick polyamide layers. The process is also limited to water-soluble amine monomers, narrowing the scope for engineering membrane hydrophilicity, hydrophobicity, and microstructure.

The strategy incorporates three major innovations. First, introducing a diphenyl ether moiety into the diamine monomer balances hydrophilic and hydrophobic domains within the polyamide layer, creating Janus-like dual transport pathways—one favoring polar solvents and the other non-polar solvents—thus enabling rapid permeation across a broad solvent polarity range. Second, embedding a contorted structural unit into the polyamide backbone increases intrinsic microporosity and pore interconnectivity, significantly enhancing solvent permeability. Third, replacing water with a deep eutectic solvent (DES) broadens the choice of amine monomers to include those insoluble in water and allows precise tuning of interfacial properties and reaction conditions in an anhydrous environment, offering far greater control over the interfacial polymerization process. Together, these features enable the formation of ultrathin, highly microporous, and amphiphilic polyamide layers, yielding TFC membranes with exceptional performance and Janus-like transport characteristics.
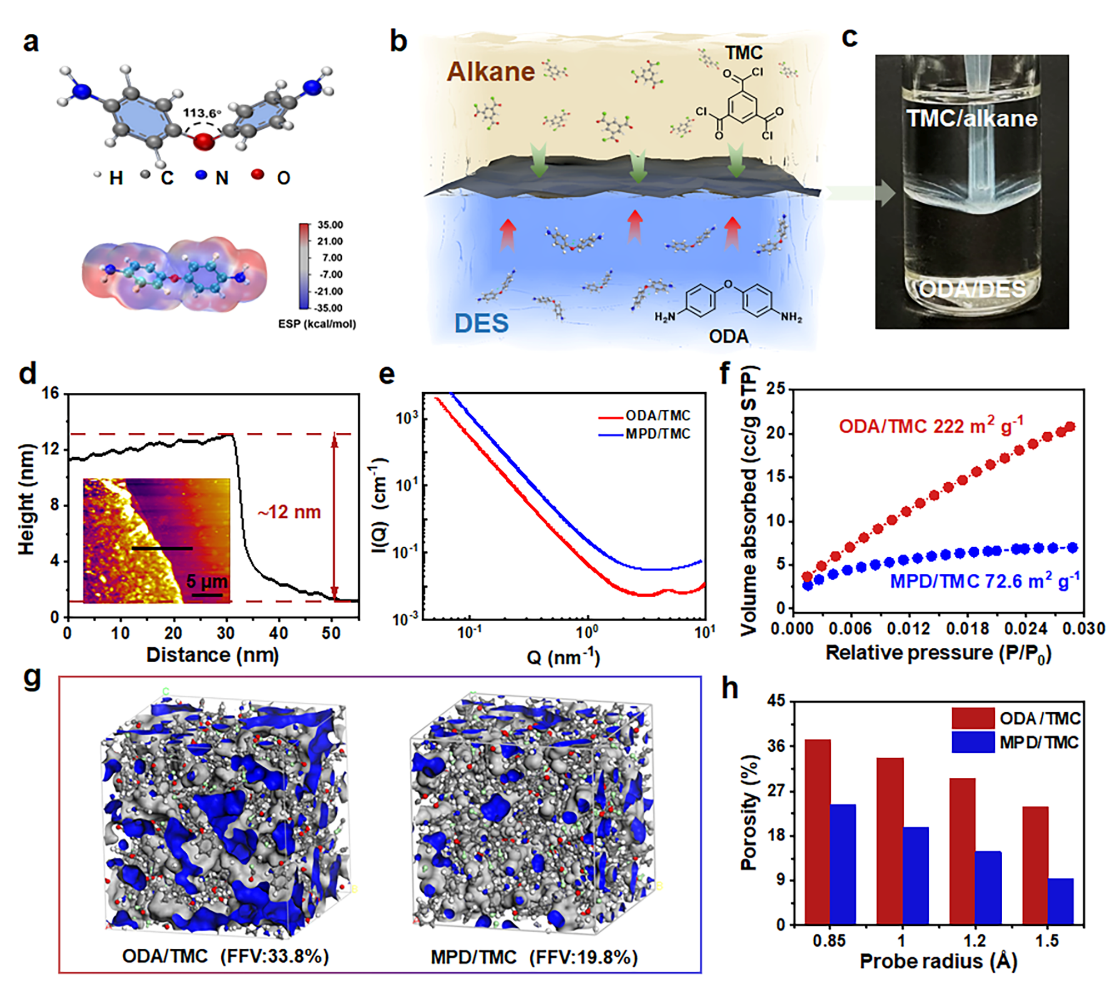
Fig.1: ODA/TMC membrane formation and characterization.
A dense, defect-free polyamide composite membrane is created through in-situ interfacial polymerization on a nylon substrate. The resulting membrane surface shows strong affinity for organic solvents, exhibiting contact angles below 25° for polar solvents and even lower values—under 10°—for non-polar solvents. Its ultra-low critical surface tension allows solvents with lower surface tension to fully wet the membrane surface, generating a positive capillary driving force that significantly enhances liquid transport.
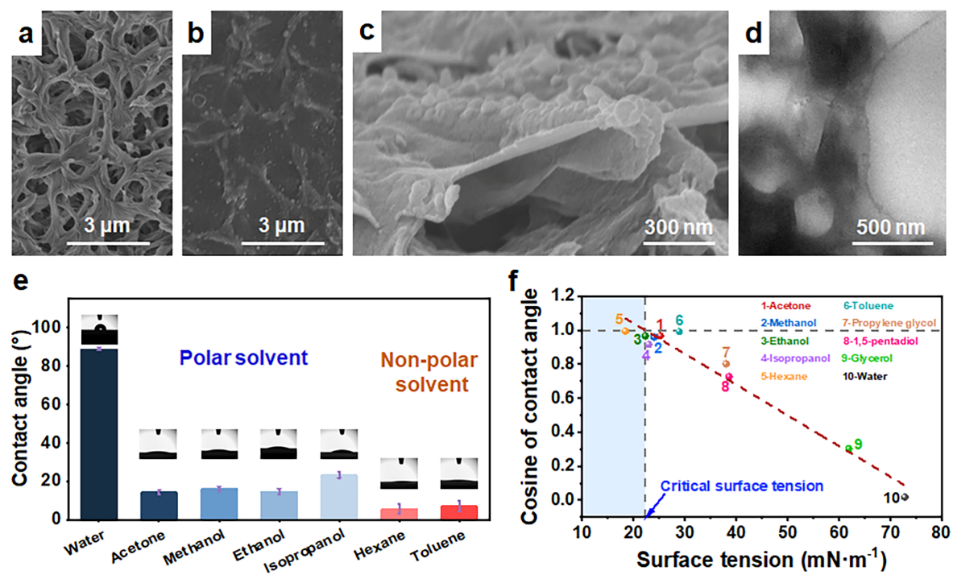
Fig. 2: ODA/TMC membrane morphologies and surface properties.
By optimizing the reaction time, the researchers fabricated highly cross-linked, ultrathin polyamide separation membranes capable of delivering both high flux and high rejection. The steep rejection profile and the narrow gap between the Molecular Weight Retention Onset (MWRO) and the Molecular Weight Cut-Off (MWCO) indicate a remarkably uniform pore structure and precise molecular sieving performance, allowing efficient separation of molecules with similar sizes. The membrane demonstrates exceptionally high permeance for both polar and non-polar solvents, underscoring its significant performance advantage. Solvent transport is influenced by multiple factors—including viscosity, solvent–membrane interactions, and steric hindrance—working together to determine overall transport behavior.
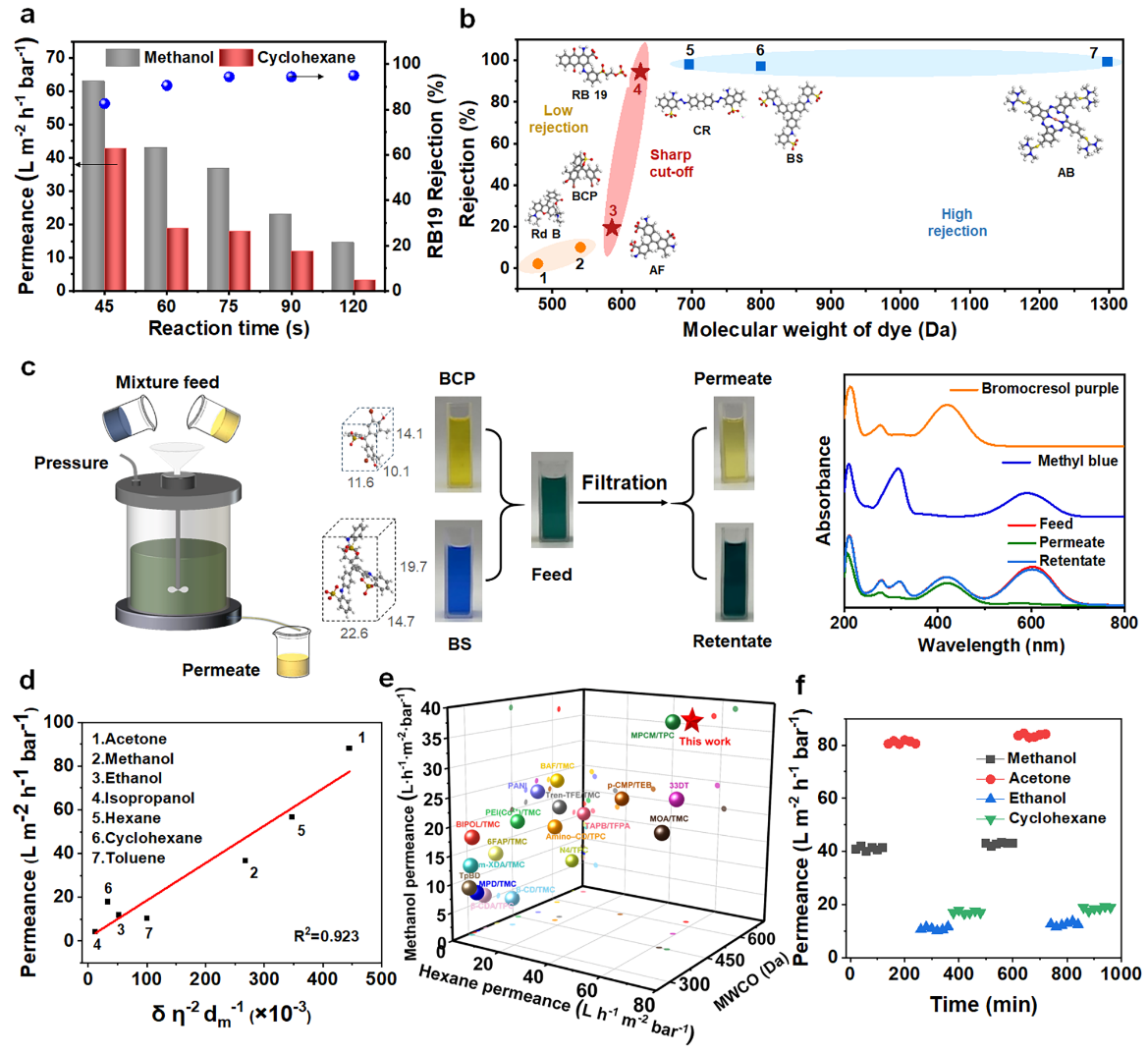
Fig. 3: Nanofiltraion performance.
Solvent transport through the ODA/TMC membrane is a multifaceted process, examined in detail through a serial resistance model combined with molecular dynamics simulations. Analysis using the Dagan model shows that, for ultrathin and densely cross-linked membranes, internal pore resistance remains the dominant barrier, yet entrance resistance also plays a meaningful role. The Lucas–Washburn equation further suggests that a positive capillary driving force can effectively reduce this entrance resistance, thereby improving overall transport efficiency. Molecular dynamics simulations support these conclusions, revealing that n-hexane permeates the membrane more rapidly than methanol, consistent with the experimental observations.
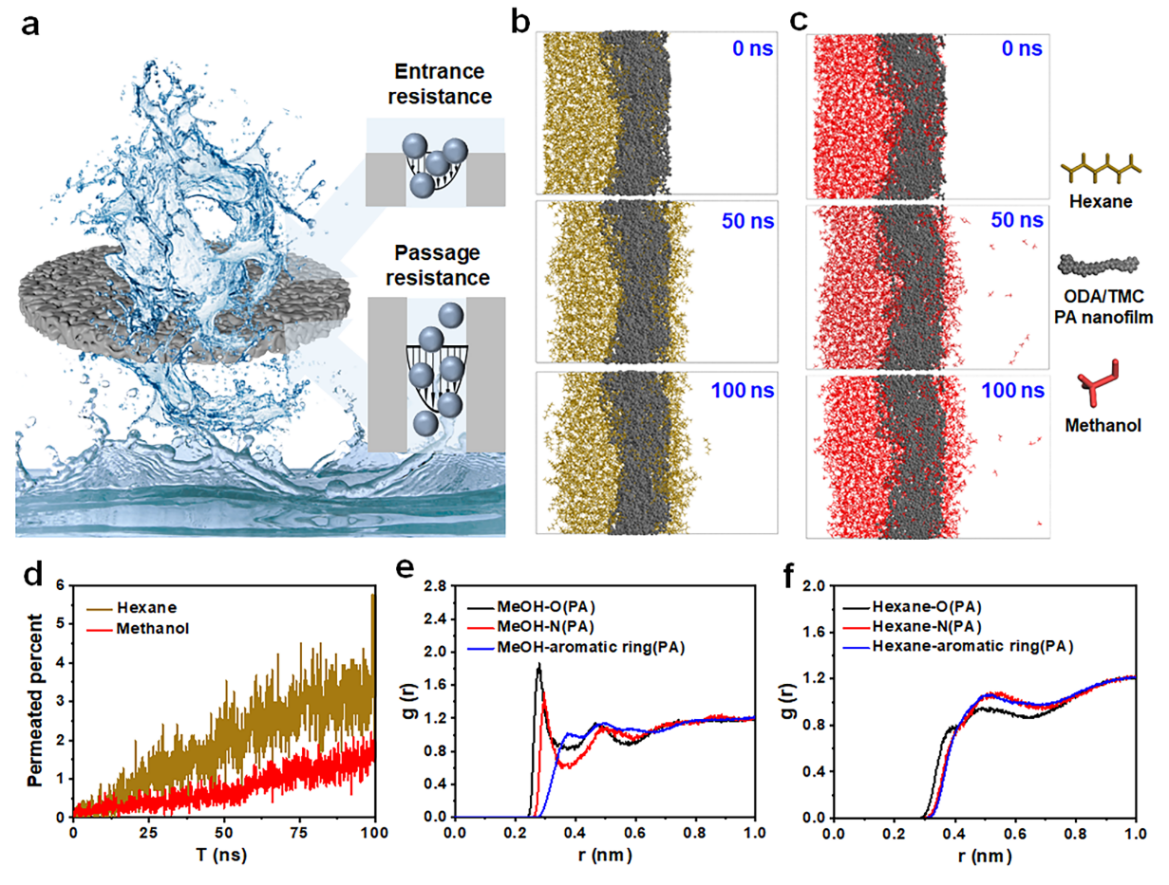
Fig. 4: Solvent transport behaviors.
Paper Link: https://www.nature.com/articles/s41467-025-63663-0


