Aqueous zinc-ion batteries (ZIBs) have attracted significant attention due to their high safety, environmental friendliness, and low cost. Among them, aqueous zinc-iodine batteries (Zn–I₂) are considered highly promising energy storage systems owing to the abundance of iodine resources and high reversibility. However, the conventional two-electron reaction (I⁻/I⁰) only provides a theoretical specific capacity of 211 mA h g⁻¹, limiting the energy density. Introducing the four-electron conversion mechanism involving the I⁰/I⁺ conversion can significantly enhance the capacity and voltage, and it is a key approach to achieving high-energy-density storage. Although high concentrations of halides facilitate the activation of the I⁰/I⁺ conversion, challenges such as zinc anode corrosion, I⁺ species hydrolysis, and the sluggish reaction kinetics still remain. Recent studies show that protons can improve iodine redox kinetics and suppress side reactions, yet excess free protons exacerbate anode corrosion. Therefore, constructing an electrolyte that combines ion regulation and interface protection functions to achieve a balance between accelerating iodine conversion and preserving anode stability is essential to high-performance four-electron Zn–I₂ batteries.

Recently, the team led by Professor Wu Peiyi and Researcher Jiao Yucong from the College of Chemistry and Chemical Engineering, DHU proposed a new electrolyte regulation strategy based on an acidic deep eutectic solvent (ZPDES). The deep eutectic solvent was formed with the mixture of high-concentration zinc chloride and phosphoric acid in this study (Figure 1).
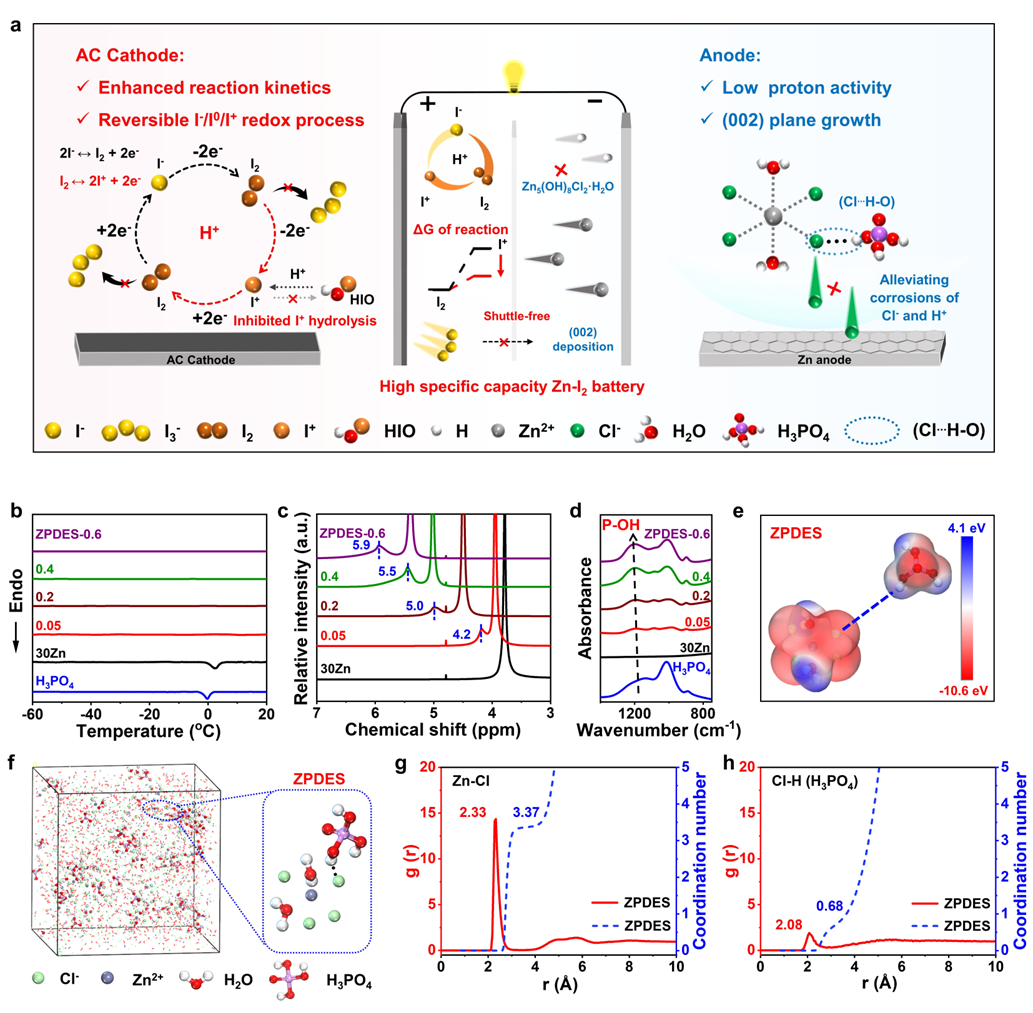
Figure 1. Structure and interaction characterizations
ZPDES significantly widens the electrochemical stability window (ESW) of electrolytes and exhibits excellent anti-corrosion performance. This electrolyte effectively suppresses the generation of by-products and promotes the preferential and oriented growth of the Zn (002) crystal plane, thereby enhancing the interfacial flatness and cycling stability of the zinc anode. Theoretical calculation results further verify the key role of proton modulation in crystal plane orientation (Figure 2).
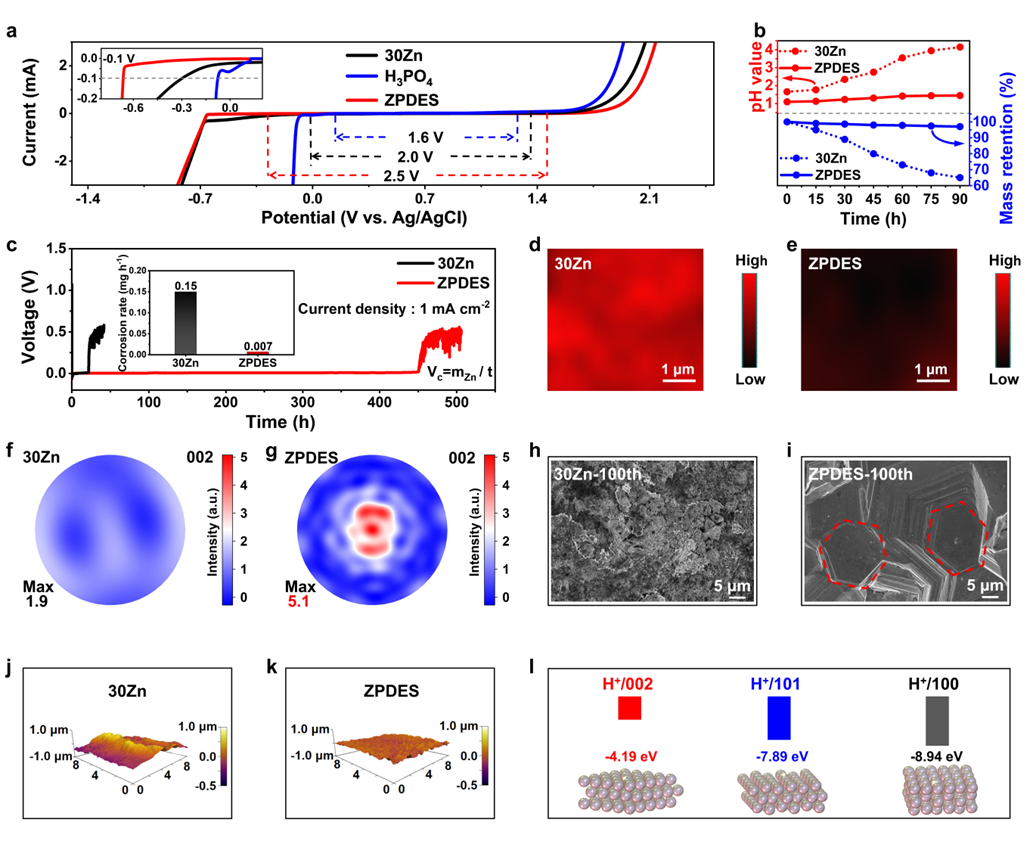
Figure 2. Corrosion inhibition performances
A reversible four-electron reaction was achieved in the ZPDES system. This system accelerates the redox reaction kinetics of I₂, significantly reduces the energy barrier for I⁰/I⁺ conversion, and simultaneously suppresses I₃⁻ generation and I⁺ hydrolysis, thereby promoting multi-electron conversion and enhancing reaction reversibility (Figure 3).
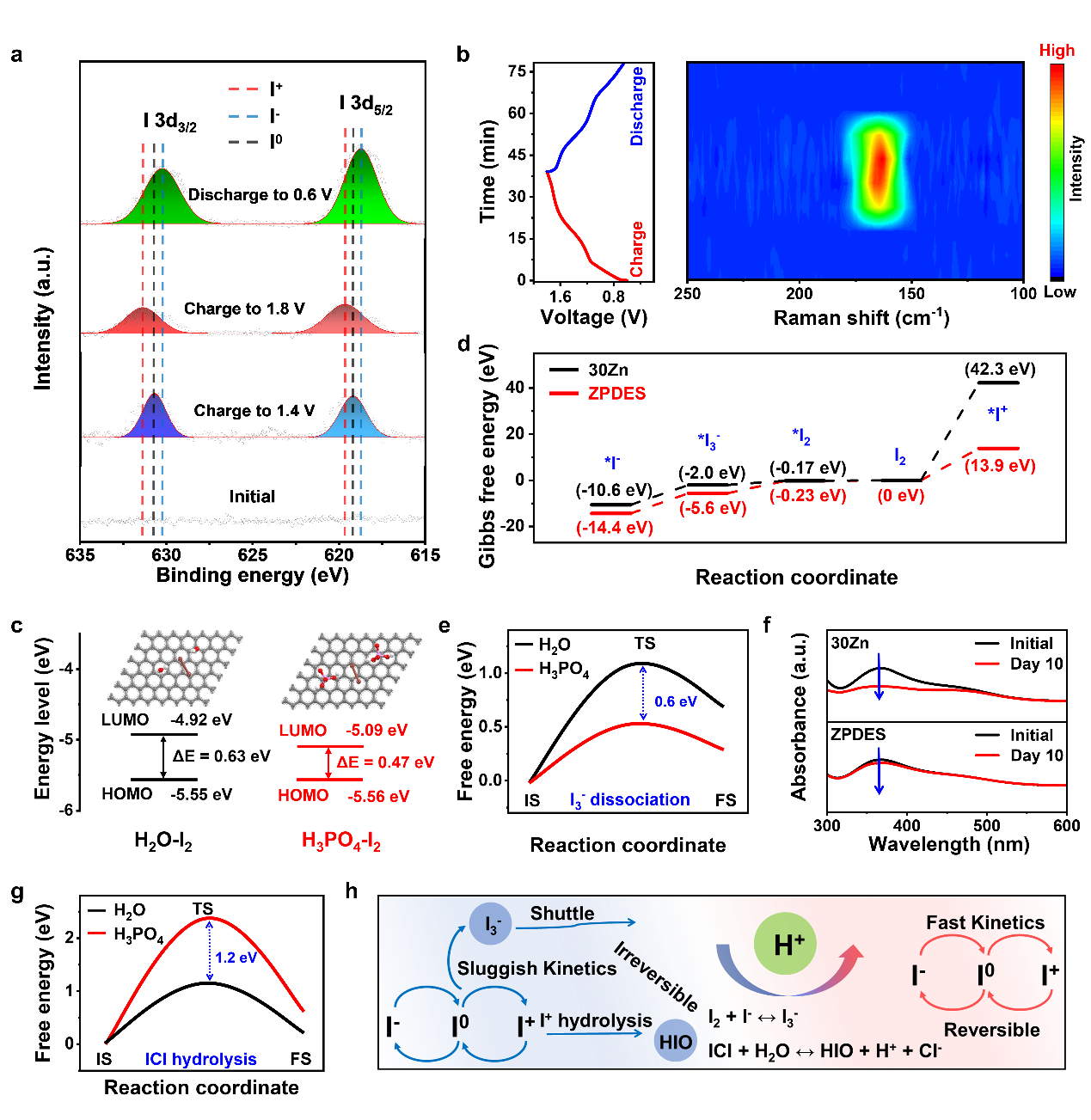
Figure 3. Proton-enhanced redox mechanism investigation
The ZPDES system exhibits lower polarization voltage and higher specific capacity, contributing to excellent redox kinetics. This system effectively reduces the redox energy barrier, accelerates the Zn²⁺ diffusion rate, and significantly decreases the charge transfer resistance, demonstrating efficient ion transport and electrode reaction characteristics (Figure 4).
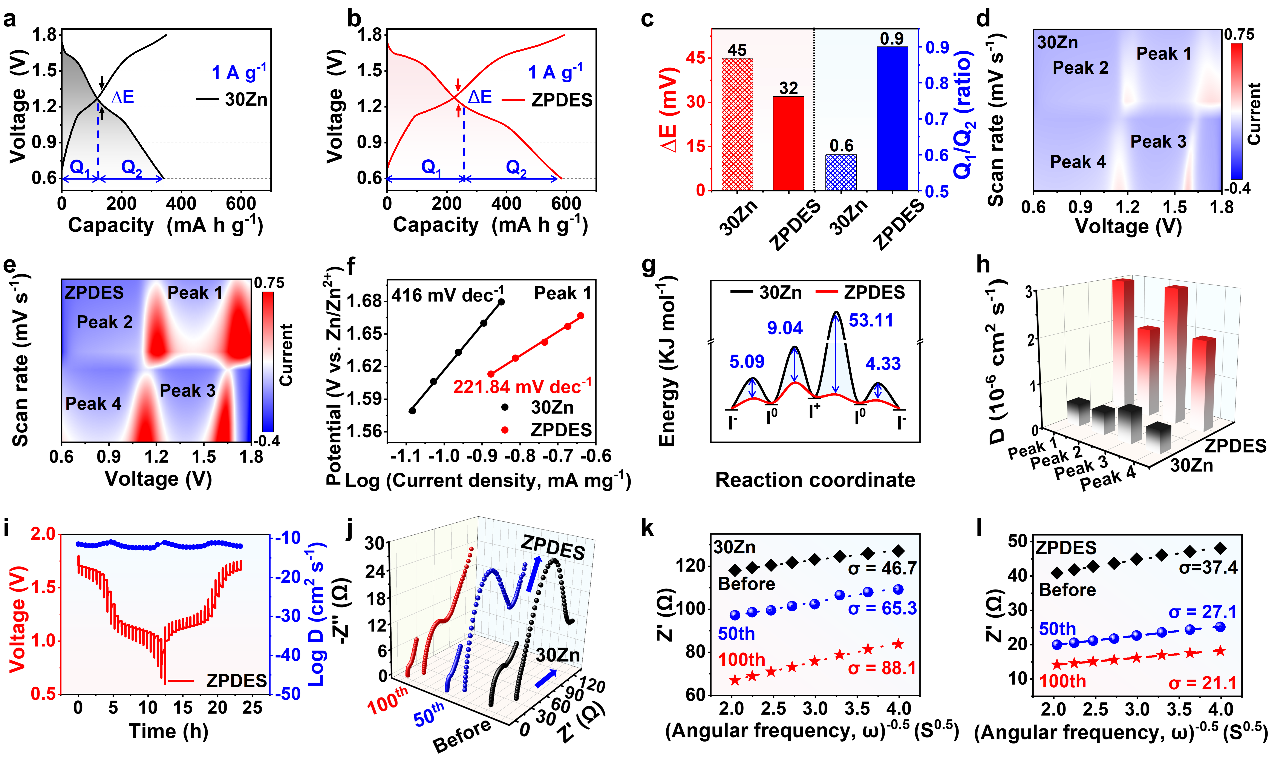
Figure 4. Redox kinetics characterization of four-electron Zn–I₂ battery
Benefiting from the excellent anti-corrosion characteristics of ZPDES and its ability to promote redox reactions, the Zn–I₂ full battery constructed with this electrolyte demonstrates outstanding cycling stability. It exhibits high specific capacity and capacity retention at different current densities, further proving the potential advantages of ZPDES in practical applications (Figure 5).
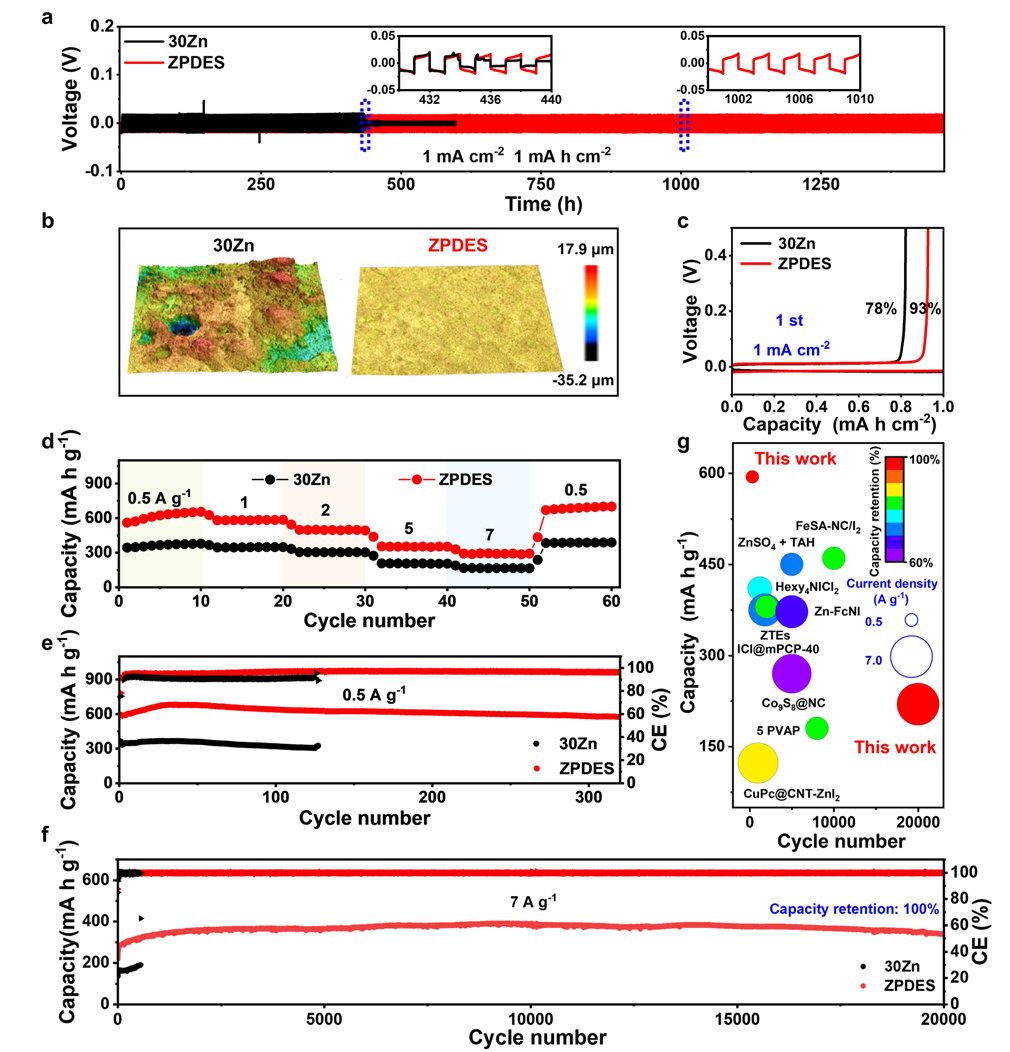
Figure 5. Battery performance evaluation
The Master’s student Yan Yuhuan from the College of Chemistry and Chemical Engineering, DHU is the first author of the paper. The corresponding authors are Researcher Jiao Yucong and Professor Wu Peiyi. The related findings were recently published in Angewandte Chemie International Edition titled Realizing High-Performance Four-Electron Zinc–Iodine Batteries with Acidic Eutectic Electrolyte. This work was supported by the National Natural Science Foundation of China, the Fundamental Research Funds for the Central Universities, and the Natural Science Foundation of Shanghai.


