Piezoelectric materials have attracted increasing interest in neuroscience research due to their unique electromechanical conversion properties. Particularly in promoting the orientation of neural cells, piezoelectric materials can provide non-invasive electrical stimulation (ES), promoting nerve injury repair. However, current natural piezoelectric biomaterials, such as silk fibroin (SF), generally lack periodic piezoelectric domains and generate a non-directional electric field, thus limiting neurite growth to a specific orientation.Furthermore, the difficulty of visualizing the electric field distribution of traditional piezopolymers prevents researchers from observing its real-time effects on neurites.
To address this issue, the team led by Professor Zhang Yaopengand Researcher Fan Sunafrom the State Key Laboratory of Advanced Fiber Materials and the College of Materials Science and Engineering, DHU, successfully fabricated visible periodic piezoelectric domains in SF films with a photochemistry strategy. The micron-level distinguishable periodic piezoelectric domains were composed of in situ synthesis of silver nanoparticles (AgNPs) within the SF film. These AgNPs were simultaneously used as a developer and mediator to precisely regulate the polarization charge (d33) and voltage (g33). The existence of periodic piezoelectric domains and the periodic electric field distribution were confirmed by piezoresponse force microscopy, electrostatic force microscopy, and amplitude-modulated Kelvin probe force microscopy (PFM, EFM, and AM-KPFM). Under ultrasound stimulation(US), the SF piezoelectric generator achieved maximum root mean square current, energy density, and voltage of 5.1 mA, 6.7 W m⁻², and 529.5 mV, respectively. It was demonstrated that the fabricated periodic piezoelectric domains remarkably regulated rat pheochromocytoma cells (PC12) neurite directional growth, length, and gene expression, and enabled the timely observation of the electric field’s effect on neurites by biological microscopy. This research, titled Visible Periodic Piezoelectric Domains in Silk Fibroin for Neurite-Orientated Extension,was published online inAdvanced Materials. The first author of the paper is Chen Jie, a DHU PhD student.

Visible Periodic Piezoelectric Domains in SF Films
The patterned SF film was fabricated by immersing it in silver nitrate solution, followed by exposure to LED light under the protection of a patterned photomask, resulting in developing patterns distributed on the surface and inside the film. This method enabled the production of SF film with a precise patternat the micrometer scale (Figure 1). Researchers adopted X-ray photoelectron spectroscopy to characterize the composition and distribution of patterned films and confirmed via transmission electron microscopy (TEM) that AgNPs with lattice spacings of 2.0 and 2.3 Å were distributed in the patterned regions. The above results confirmed that the patterned film in this study was a composite SF/AgNP material.
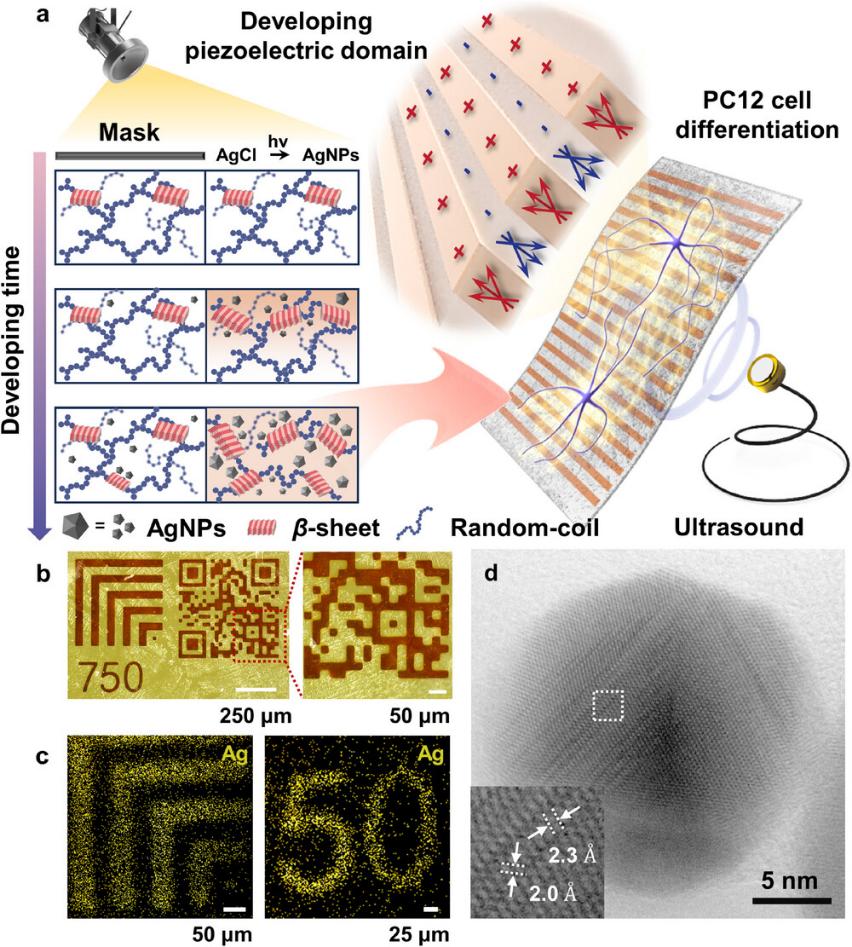
Figure 1. Patterned film fabricated by the photochemistry method. a) US-driven piezoelectric patterned film used for PC12 differentiation. Interval patterns were fabricated on SF film using photochemical strategies. b,c) Optical microscope images (b) and XPS mapping (c) of the patterned film. d) TEM image of AgNPs extracted from the patterned film.
Periodic Piezoelectric Domains
Significant piezoelectricity differences were observed between the patterned and patternless regions of the film (Figure 2). The patterned SF film surface exhibited a dendritic morphology. The amplitude and phase feedback were different from the surface morphology and showed alternating dark and bright lines with evident color differences, where the patterned region was brighter in color. This suggested that the patterned region possessed opposite domains to those of the patternless region. The piezoelectric coefficient is closely related to the material structure. The crystallinity of the patterned and patternless regions were studied with synchrotron radiation wide-angle X-ray diffraction. The patterned region showed a peak of AgNPs at 18.96。(d-spacing: 2.35, Q: 2.67), corresponding to the (111) lattice plane, and showed a higher crystallinity (60%) compared to that of the patternless region, indicating that more non-centrosymmetric crystals were formed (mainly β-sheet form). The in situ synthesis of AgNPs promoted secondary structure mobilityand distorted the crystal lattice. This process effectively regulated the condensed-state structure of the patterned SF film, ultimately favoring an enhancement of the piezoelectric coefficient.
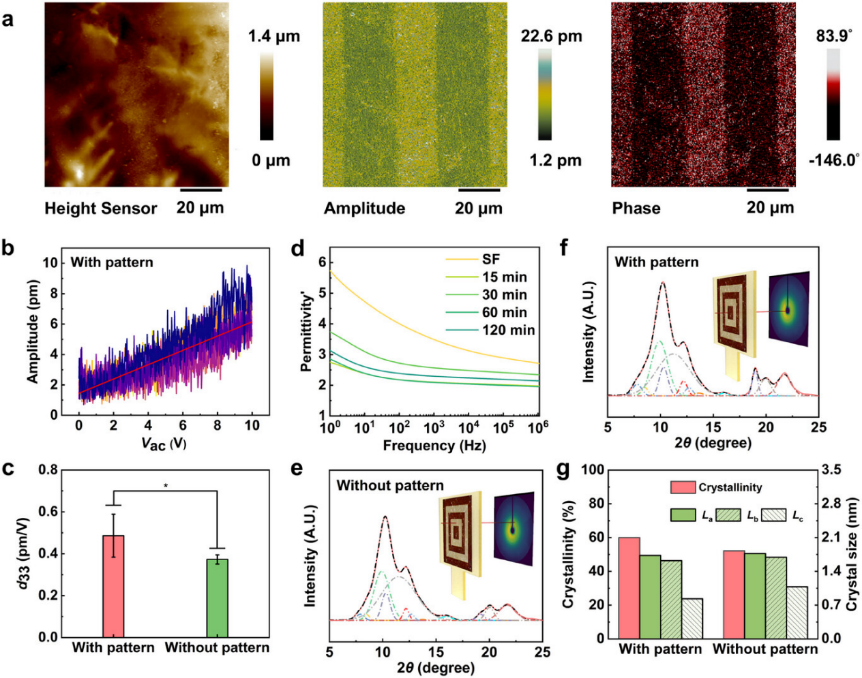
Figure 2. Difference in the electric and structure properties between patterned and patternless film regions in patterned film. a) Topography, out-of plane amplitude, and phase of the patterned film by PFM images. b) Vac dependence on piezo-response in the patterned region. The red line represents the fitting curve indicating the average of the d33 slope. c) Average d33 calculated to compare the difference between patterned and patternless regions. d) Impact of the development time on real permittivity. e,f) 1D SR-WAXD diffractograms of the patternless (e) and patterned (f) region. g) Crystallinity, and crystal size differences between patterned and patternless regions.
Electrical Output of US-Driven SF Piezoelectric Films
The US is a convenient and efficient tool for driving the piezoelectric generators (PEGs) device in liquid. SF or SF/AgNP film was integrated into PEGs and immersed in a self-made US equipment to evaluate the electric output properties in water (Figure 3). This equipment transmitted a rectangular wave of 0.6 W cm−2 and used a 1 MHz US probe, resulting in the maximum RMS voltage, current, and power of 529.5 mV, 5.1 mA, and 2.7 mW, respectively, in which the power density was equal to 6.7 W m−2. Comparatively, the PEGs showed the advantage of higher current output (Irms) and power density, meeting the requirement of electric stimulation for cell biology.
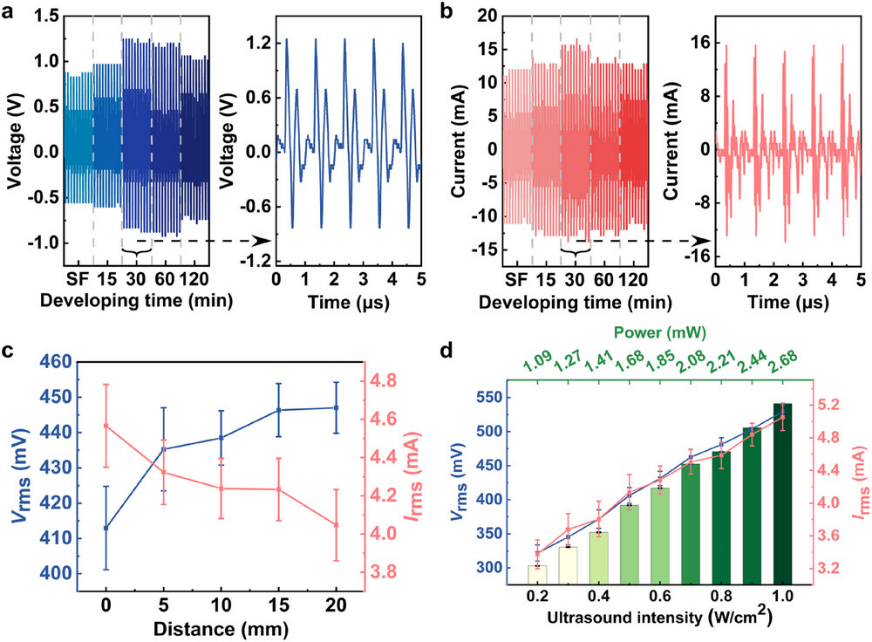
Figure 3. The piezoelectric output of SF/AgNP films under US stimulation in water. a,b) Voltage (a) and current (b) output of the films under different developing times. c,d) Effects of distance (c) and US intensity (d) on the root mean square value of the piezoelectric output of the SF/AgNP film.
Neurite-Orientated Extension and Gene Expression on Periodic Piezoelectric Domains
Although the use of AgNPs for medical devices is approved, the toxicity of full-exposure SF/AgNP film on PC12 must still be carefully evaluated (Figure 4). PC12 on SF/AgNP film showed a decreased viability compared to that of PC12 on pristine SF film. However, PC12 viability still increased by 588% on SF/AgNP film on day 5 compared to day 1. Hence, patterned films could be used for the subsequent experiment on PC12 differentiation. Subsequently, a film with a 10 µm interval pattern was driven by 1 MHz US to stimulate PC12 differentiation. PC12 were visible under a microscope thanks to the well-optical transparent film, and all of them had a circular shape on the film after seeding for 4 hours. After 5 days, an extension of neurites of PC12 was observed in the vertical direction of the pattern when compared to pristine SF.
To observe neurite growth on the patterned film (Figure 5), fluorescent agents were used in staining the microtubule protein of class III β-tubulin (Tuj1) and microtubule-associated protein 2 (MAP2). The neurite of PC12 cultured on the patterned film was uniformly extended in the vertical direction of the patterned lines, regardless of the US stimulation. In contrast, the PC12 neurite extended anywhere on the pristine SF film. This suggested that the pattern induced the direction of the neurite extension. The statistical analysis of the average neurite length showed that the pattern promoted an increase in length to 110.0 ± 40.1 μm, compared to the effect of pristine SF film (107.5 ± 40.7 μm). The neurite length further increased to 120.5 ± 50.0 μm under US compared to the effect of pristine SF with US (113.2 ± 40.7 μm). Furthermore, significant differences were observed in gene expression. The expression of Tuj1 and the GFAP gene under the effect of the pattern increased by 1.46-fold and 2.17-fold respectively, compared to that under the effect of pristine SF film without US stimulation. Additionally, an increase of 1.66-fold in the relative content of Tuj1 and 17.53-fold in the GFAP gene were noted on the patterned film under US stimulation compared to that on the pristine SF film without the US. The above results demonstrated that the patterned film driven by the US induced the direction of the neurite extension, accelerated neurite growth, and promoted the expression of genes involved in the formation and maintenance of synapses in neurons as well as promoting the maturation of astrocytes.
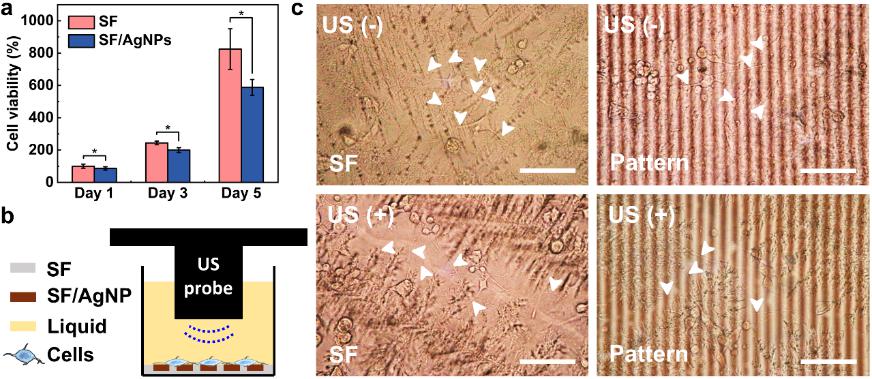
Figure 4. PC12 cell viability and differentiation. a) Viability of PC12 cells on SF and full exposure SF/AgNP film tested by CCK-8 from day 1 to day 5 (n = 5, *p < 0.05). b) Schematic diagram of US-driven stimulation for PC12. c) Optical images of PC12 neurite affected by US and pattern. The white arrows in (c) indicate the neurites. “–”: cell culture without US stimulation; “+”: cell culture with US stimulation. Scale bar: 100 μm.
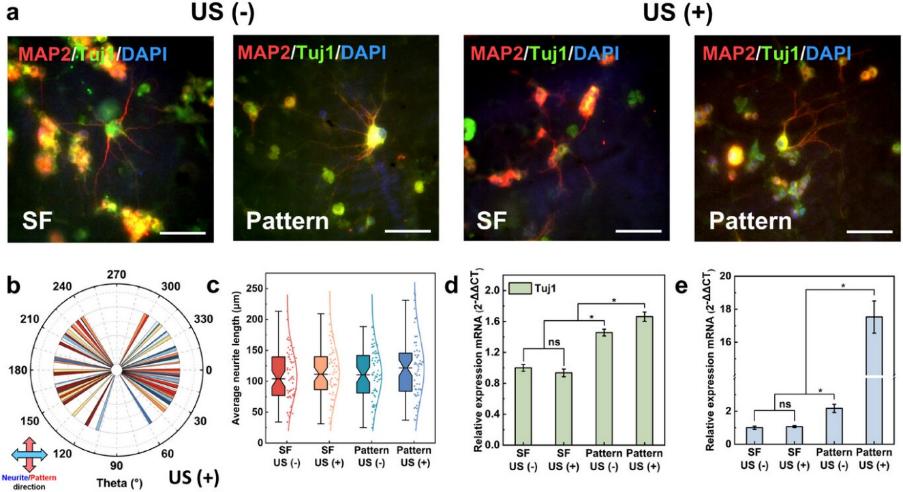
Figure 5. Differentiation of PC12 cells stimulated by patterned film. a) Expression of specific neural proteins in PC12. b) Statistical analysis of neurite angles in PC12 to quantify the orientation. c) Statistical analysis of PC12 neurite lengths. d,e) mRNA expression of a specific neural protein in PC12. “–”: cell culture without US stimulation; “+”: cell culture with US stimulation. Scale bar: 50 μm. “ns”: not-significant.
This study not only addresses the lack of periodic electric field distribution in natural piezoelectric biomaterials but also successfully achieves visualization of the electric field distribution, providing a novel tool for studying the effects of non-invasive ES on cell behavior. More importantly, this method features high efficiency and scalability for production, opening up new possibilities for its applications in cell biology.In the future, this technology is expected to play important roles in nerve injury repair, ES therapy, and bioelectronic devices, paving the way for precise ES treatments.


