A research team from Donghua University’s College of Materials Science and Engineering and the State Key Laboratory of Advanced Fibers and Polymer Materials, led by Professors Liao Yaozu and Lü Wei, has achieved a major breakthrough in the development of cathode materials for high-rate, fast-charging lithium-ion batteries (LIBs). Duan Ju, a doctoral student at Donghua University’s College of Materials Science and Engineering, is the first author of the study, with Professors Liao Yaozu and Lü Wei serving as corresponding authors. The team’s findings were recently published as a Hot Paper in Angewandte Chemie International Edition under the title “Heteroporous Donor–Acceptor Covalent Organic Framework Cathode for High-Rate-Capacity Lithium-Ion Batteries.” The team’s innovative design of heteroporous covalent organic frameworks (HCOFs) could pave the way for next-generation lightweight and high-capacity energy storage systems.
Lithium-ion batteries (LIBs) have revolutionized modern technology, powering devices and vehicles that drive social and industrial transformation. With the rapid growth of the low-altitude economy, including applications such as unmanned aerial vehicles (UAVs) and light sport aircraft, there is a rising need for lightweight, high-rate, and fast-charging energy storage systems. As the core component of LIBs, cathode materials directly determine the battery’s performance, energy density, and weight. Developing cathodes that combine low mass, rapid charge–discharge capability, and long-term stability has therefore become a pressing challenge in advanced energy research.
To address this challenge, the Donghua University team adopted a heteropore engineering strategy to design donor–acceptor (D–A) type covalent organic frameworks (HDA-COFs) using a one-pot Schiff base reaction. Heteroporous covalent organic frameworks (HCOFs)—a class of COFs characterized by kgm topology and multiple pore structures—have shown great promise as cathode materials for high-performance LIBs. Their diverse pore environments and unique size effects provide an ideal structure for achieving both efficient charge transport and high energy storage capacity. By rationally designing HCOFs to simultaneously enhance electronic conductivity (σₑ)and ion diffusion (σᵢₒₙₛ), researchers can unlock new possibilities for creating high-rate, fast-charging cathode materials that deliver exceptional performance and stability.

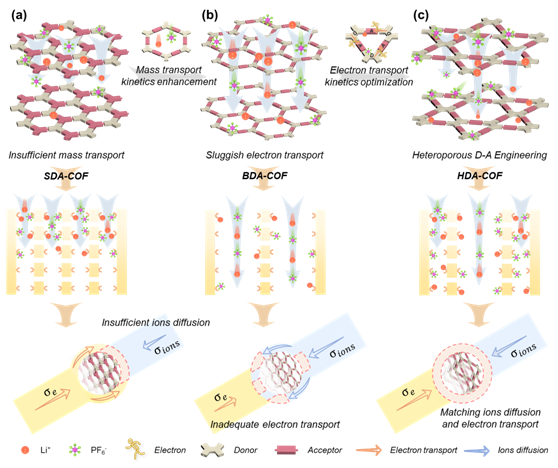
Figure 1: Schematic diagram of HDA-COF design
To highlight the superior performance of HDA-COFs, the research team synthesized a comparison sample—a covalent organic framework with a single mesopore, termed BDA-COF. Structural and morphological analyses (Figure 2) confirmed the successful synthesis of both materials through Schiff base reactions. Powder X-ray diffraction (PXRD)and structural simulations revealed distinct architectures: BDA-COF features an hcb topology with a single mesopore of 3.45 nm, whereas HDA-COF adopts a kgm topology with dual pores of 2.23 nm and 4.46 nm. Further evidence from solid-state ¹³C NMR and Fourier-transform infrared (FT-IR) spectroscopy confirmed the formation of imine bonds, validating the successful construction of both frameworks.
Nitrogen adsorption testing revealed that HDA-COF possessed an exceptional specific surface area of 922 m²/g and 51% porosity, outperforming the BDA-COF’s 224 m²/g and 13% porosity. Transmission electron microscopy (TEM) confirmed the highly crystalline hexagonal pore structure consistent with theoretical AA stacking predictions, further validating the success of the heteroporous design.
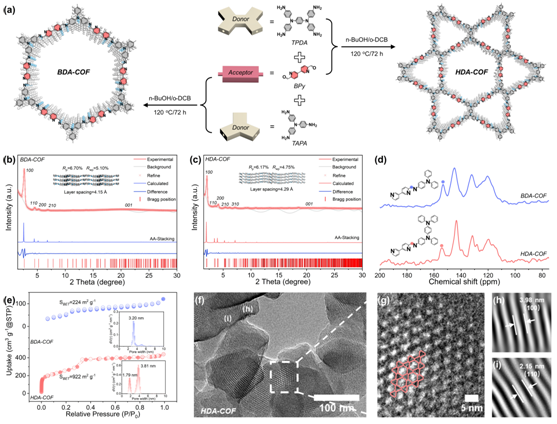
Figure 2: Structural and morphological characterizations
The team conducted density functional theory (DFT) calculations to explore the electronic structure and ion transport mechanisms of the three COF materials (Figure 3). Analyses of the molecular electrostatic potential (MESP) and HOMO–LUMO orbitals revealed distinct charge distributions: electron-rich regions (associated with imine and pyridine nitrogen atoms) favor n-type redox reactions, while electron-deficient regions (centered on triphenylamine units) promote p-type redox behavior. This complementary interaction between n-type and p-type domains imparts the materials with a bipolar charge storage capability, a key advantage for high-performance lithium-ion battery cathodes.
HDA-COF demonstrated a narrower band gap (2.29 eV) and a higher HOMO energy level (-4.77 eV), resulting in an electronic conductivity of 5.1×10⁻¹⁴ S/cm, outperforming other COF materials. The findings reveal that the heteroporous architecture of HDA-COF effectively promotes donor–acceptor (D–A)–type electron conduction, enhancing overall charge mobility. Further ion binding energy analyses showed that the hexagonal mesopores facilitate rapid ion diffusion, while the triangular micropores strengthen Li⁺ binding but moderately limit transport kinetics. Together, these dual pore structures work synergistically to achieve efficient charge transfer and balanced electrochemical performance.
By combining DFT calculations with a series of ex situ spectroscopic analyses, the research team verified the active sites and bipolar redox behavior of both BDA-COF and HDA-COF frameworks (Figure 4). The results revealed that pyridine and imine sites can coordinate with up to four Li⁺ ions during discharge, while triphenylamine units reversibly interact with PF₆⁻ anions during charging. Ex situ electron paramagnetic resonance (EPR) spectra further demonstrated reversible changes in radical signal intensity throughout charge–discharge cycles, confirming stable redox activity. Complementary FT-IR and X-ray photoelectron spectroscopy (XPS) analyses provided additional evidence of the reversible intercalation and deintercalation of C═N (n-type) and PF₆⁻ (p-type) species across both high- and low-voltage regions. Together, these findings conclusively establish a clear, reversible bipolar reaction mechanism within the COF materials, underscoring their potential for high-performance energy storage applications.
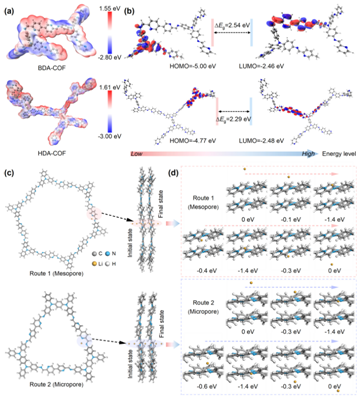
Figure 3: Electronic conduction and ion diffusion in HDA-COF
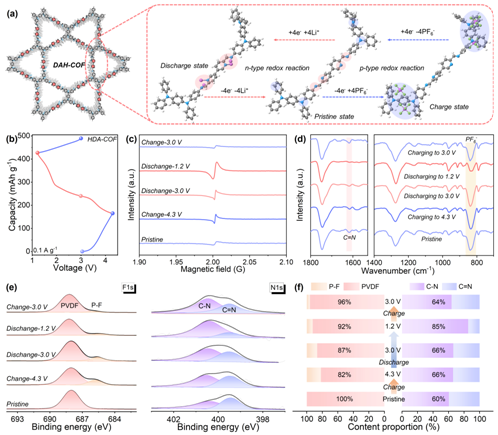
Figure 4: Charge storage mechanism
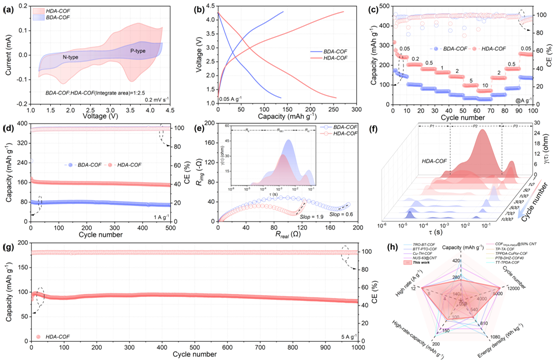
Figure 5: Electrochemical performance characterizations
Benefiting from heteroporous D-A engineering, HDA-COF demonstrated significantly enhanced electrochemical performance (Figure 5). It delivered a discharge capacity of 251 mAh g⁻¹ at 0.2 A g⁻¹, surpassing BDA-COF (220 mAh g⁻¹), and maintained 71 mAh g⁻¹ even at 10 A g⁻¹, demonstrating excellent rate capability. After 500 charge–discharge cycles, HDA-COF retained 85% of its capacity, far exceeding the 49% retention observed for BDA-COF. Electrochemical impedance spectroscopy (EIS) revealed a lower charge-transfer resistance (11.5 Ω) and a higher ion diffusion rate, confirming that the heteroporous framework effectively enhances electron/ion transportand reaction reversibility, thereby ensuring long-cycle stability and high-rate performance, even under heavy loading conditions.
Further analysis (Figure 6) showed that heteroporous D–A engineering also improved charge and ion transport behavior under high mass loading. The surface-controlled capacitance contribution of HDA-COF reached 78%, higher than BDA-COF’s 73%, while its ion diffusion coefficient (~7.9 × 10⁻¹⁰ cm² s⁻¹) and charge-transfer rate (~4.1 × 10⁻⁶ mol s⁻¹ m⁻²) were both superior. Moreover, its lower charge-transfer resistance (44 Ω) and activation energy (0.50 eV) indicate that the heteroporous architecture facilitates electrolyte penetration and active-site accessibility. Together, these features synergistically enhance both surface- and diffusion-controlled capacitance, achieving an optimal balance between electron transport and ion diffusion for high-efficiency energy storage.
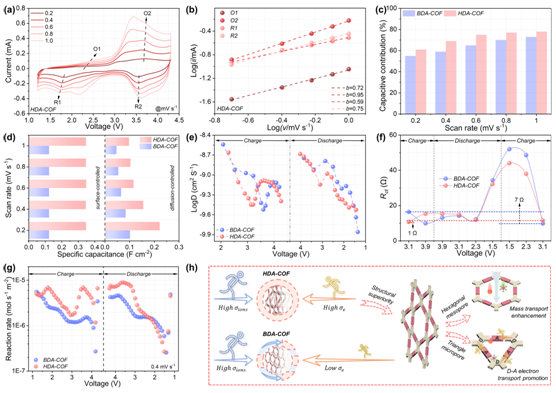
Figure 6: Ion diffusion and charge transfer behavior
The research not only advances the understanding of COF-based electrochemistry but also opens new pathways for designing lightweight, high-capacity, and durable energy storage systems.


