A research team from Donghua University (DHU), led by Professor Lingling Chu of the Advanced Low-Dimensional Materials Center, in collaboration with Professor Bruce A. Arndtsen’s group at McGill University (Canada), has achieved a major breakthrough in the field of enantioselective carbonylative coupling reactions.
The team’s findings were recently published in Journal of the American Chemical Society (JACS), one of the most prestigious journals in the field. Li Ling, a doctoral student at DHU, is the first author, and DHU is listed as the first corresponding institution.

Transition metal-catalyzed carbonylative coupling reactions are fundamental to the synthesis of pharmaceuticals, natural products, and functional materials. However, constructing α-carbonyl chiral centers, which are widely present in bioactive compounds, has remained a persistent challenge, particularly when forming them through asymmetric carbonylative coupling between alkyl halides and nucleophiles.
This difficulty arises primarily from the strong coordination between carbon monoxide (CO) and nickel catalysts, which inhibits the activation of C(sp³)–halides and diminishes the ability of chiral ligands to control enantioselectivity.
To tackle this issue, the DHU–McGill team developed a dual catalysis strategy that merges photoredox catalysis with chiral nickel catalysis. This innovative approach successfully achieved the first asymmetric carbonylative coupling of benzylic and related C(sp³)–halides with amines, producing a diverse range of chiral amides with excellent enantioselectivity.
In this system, the photocatalyst precisely controls the reaction’s activation and rate, while the chiral nickel catalyst governs the stereochemical outcome. This synergistic design effectively overcomes the inhibitory effects of CO on oxidative addition — a long-standing limitation in traditional nickel-catalyzed carbonylation reactions. Through careful tuning of ligands and reaction conditions, the team achieved high yield and enantiomeric excess, demonstrating both efficiency and precision.
The synthetic method developed in this study can be applied to the production of pharmaceutical compounds, including non-steroidal anti-inflammatory drugs (NSAIDs), and holds significant potential for advancing both synthetic chemistry and drug discovery.
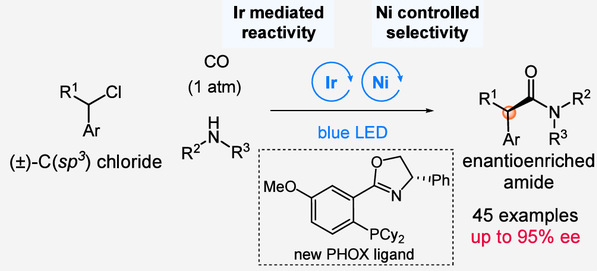
(Dual catalysis strategy enables asymmetric carbonylative coupling reactions)


