A research team led by Researcher Zhang Guojun, a Member of the World Academy of Ceramics, from the State Key Laboratory of Advanced Fibers and Materials and the Functional Materials Research Center at Donghua University (DHU), in collaboration with Researcher Wang Wenzhong’s team from the Shanghai Institute of Ceramics, Chinese Academy of Sciences, has achieved a groundbreaking advance in the partial oxidation of methane (POM), realizing the reaction under ambient temperature and pressure without a catalyst. Their pioneering study introduces a mechanical stirring–enhanced ultrasonic cavitation strategy, establishing a new, catalyst-free pathway for methane conversion and offering fresh insights into the sustainable utilization of natural gas.
In this study, the research team developed a catalyst-free partial oxidation of methane (POM) system by integrating mechanical stirring with a low-frequency ultrasonic field. Operating under ambient conditions (298 K, PCH₄ = 0.1 bar, PO₂ = 0.1 bar, PN₂ = 0.8 bar), the system achieved an impressive C₁ chemical production rate of 129.26 µmol·h⁻¹and a methane conversion rate of 22%. Mechanistic investigations revealed that introducing mechanical stirring lowers the cavitation bubble formation threshold, prevents bubble aggregation, and amplifies the ultrasonic field intensity. The resulting enhanced cavitation effect facilitates the cleavage of C–H bonds in methane and improves the participation of oxygen molecules during the reaction. This innovative approach establishes a catalyst-free pathway for methane oxidation, offering new insights into the efficient and sustainable utilization of natural gas. The researchers believe that this ultrasonic cavitation-based strategy could be extended to other energy conversion systems, marking a promising step toward greener chemical transformation technologies.
The team’s findings were published online on August 13, 2025, in the international journal Nature Communications(2025, 16, 7506) under the title “Catalyst-Free Partial Oxidation of Methane Under Ambient Conditions Boosted by Mechanical Stirring-Enhanced Ultrasonic Cavitation.” Pan Yingtong, a doctoral candidate at Donghua University’s College of Materials Science and Engineering, is the first author, while Researchers Zhang Guojun and Wang Wenzhong serve as the corresponding authors.

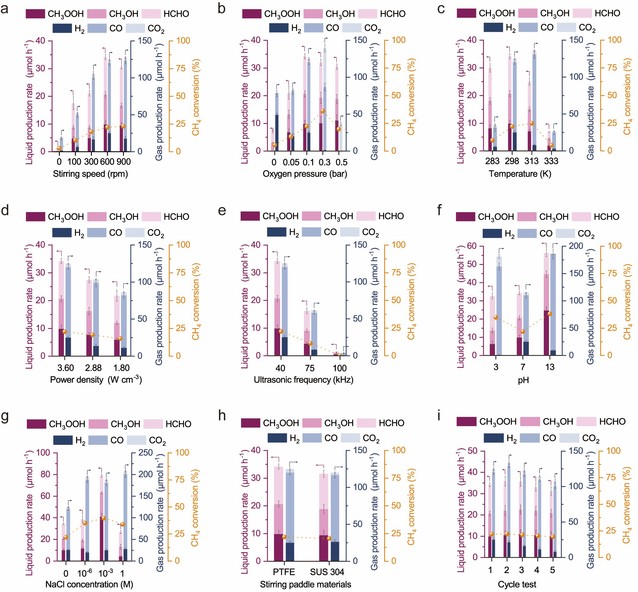
Figure 1: Product yield of POM and CH₄ conversion rate under different conditions
Figure 1 illustrates the product yield and CH₄ conversion rate under varying conditions, identifying optimal reaction parameters: 298 K, PCH₄ = 0.1 bar, PO₂ = 0.1 bar, PN₂ = 0.8 bar, ultrasonic frequency = 40 kHz, power density = 3.6 W·cm⁻³, and stirring speed = 600 rpm.
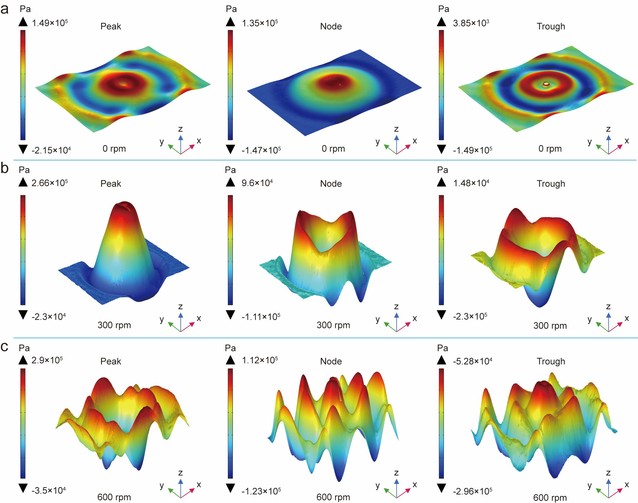
Figure 2: Acoustic field distribution under different cross-sections and stirring speeds
Figure 2 presents finite element simulations of acoustic field distributions across various stirring speeds (0, 300, and 600 rpm). The results demonstrate that at 600 rpm, the acoustic amplitude reaches its maximum, leading to a more uniform acoustic field and enhanced cavitation intensity, thus explaining the improved methane conversion efficiency.
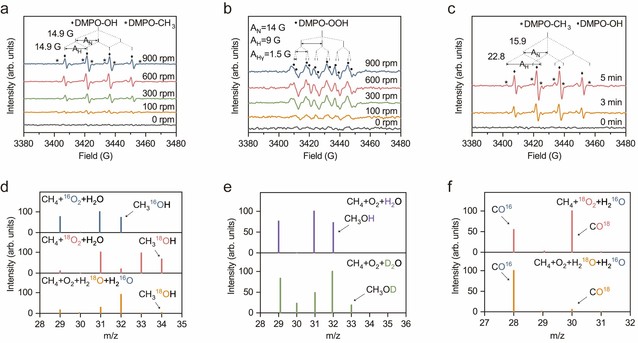
Figure 3: Mechanistic study of POM
Figure 3 provides EPR (Electron Paramagnetic Resonance) and isotope tracing (¹⁸O₂, D₂O, and H₂¹⁸O) analyses, which confirmed the formation of active radicals and highlighted the vital role of oxygen participation in the reaction mechanism.
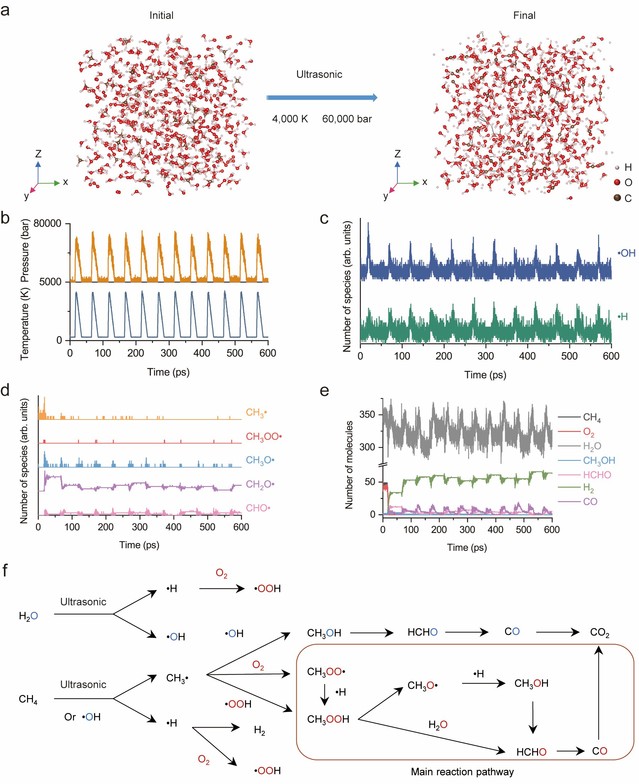
Figure 4: Molecular dynamics simulation and reaction path investigation of the POM process
Figure 4 combines molecular dynamics simulations and reaction pathway analysis, showing that the main pathway involves the formation of methyl hydroperoxide (CH₃OOH), which subsequently decomposes to produce C₁ chemicals such as methanol (CH₃OH), formaldehyde (HCHO), and carbon monoxide (CO).
Unlike traditional methane conversion methods that depend on high-temperature catalysis and energy-intensive processes, this study introduces an ingenious approach that couples mechanical stirring with low-frequency ultrasonic cavitation. This innovative system achieves the partial oxidation of methane into C₁ chemicals—such as methanol and formaldehyde—under ambient temperature and pressure, and entirely without catalysts. The breakthrough provides a sustainable, low-energy pathway for the high-value utilization of methane, offering new possibilities for cleaner and more efficient natural gas conversion technologies.


