The research team led by Professor Li Jingchao at the College of Biological Science and Medical Engineering of Donghua University has made significant progress in developing precision cancer therapies through nanomaterial engineering. Their work centers on enhancing the therapeutic efficacy and targeting precision of conjugated polymer-based systems for oncology applications. Recent breakthroughs include innovative strategies for bone metastasis therapy, pancreatic cancer treatment, and controlled gene editing.
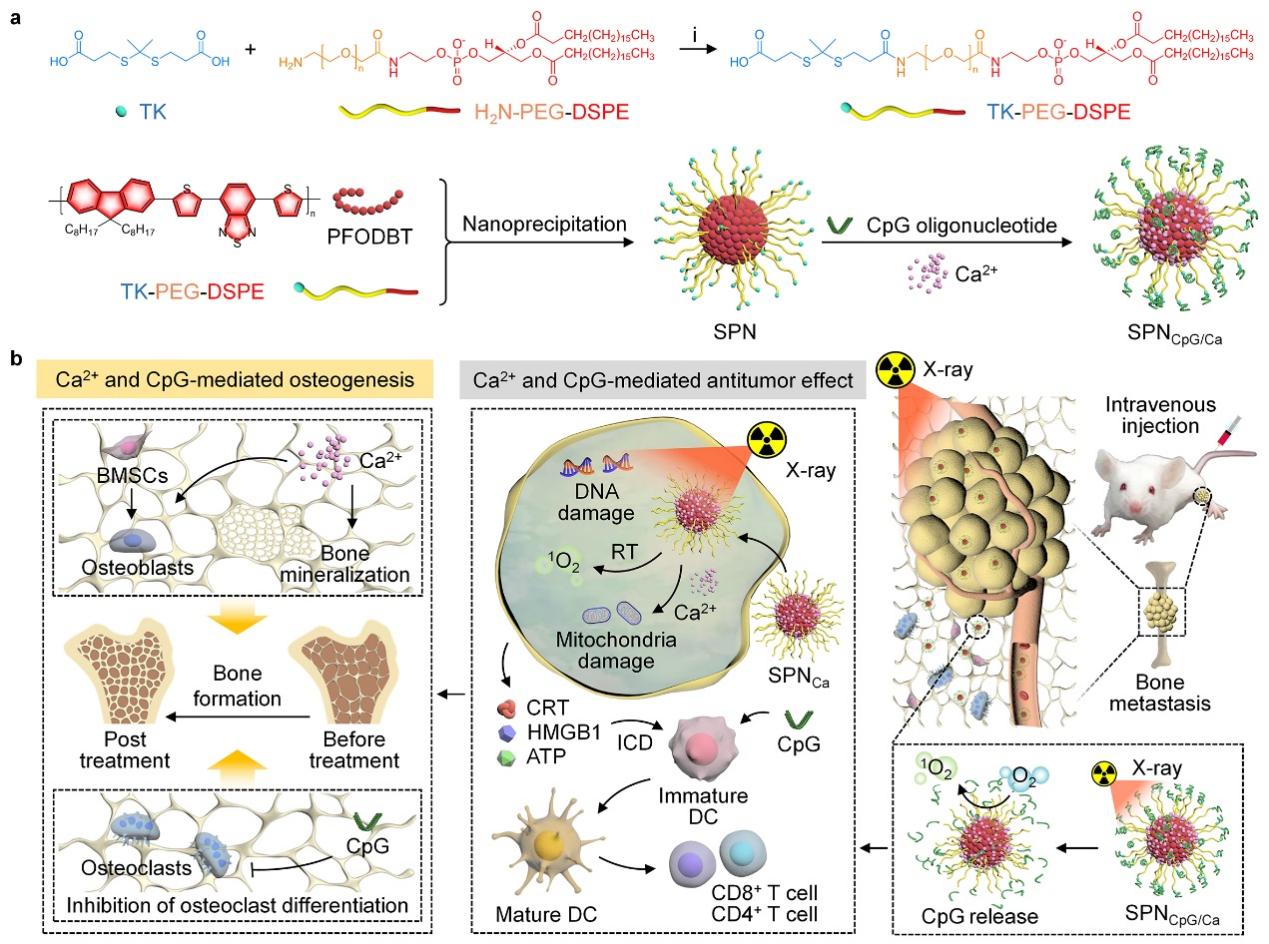
(Figure 1. Schematic diagram of the synthesis route and therapeutic mechanism of SPNCpG/Ca)
In tackling the challenge of bone metastasis treatment, the team designed a multifunctional semiconducting nanointegrator (SPNCpG/Ca) that synergizes radiotherapy, calcium overload, and immunotherapy.The system features a semiconducting polymer nanoparticle (SPN) core that functions as both a radiosensitizer and a 1O2 generator under X-ray irradiation. The reactive oxygen species produced not only exert direct radiotherapeutic effects but also cleave thioketal linkers to release immunoadjuvant CpG oligonucleotides. This coordinated mechanism induces immunogenic cell death and activates antitumor immunity through dendritic cell maturation. In breast cancer bone metastasis models, this platform achieved complete tumor regression and suppressed hepatic and pulmonary metastases. Moreover, the released Ca2+ promoted osteogenic differentiation of bone marrow mesenchymal stem cells, while CpG oligonucleotides inhibited osteoclastogenesis, collectively contributing to bone tissue regeneration (Fig. 1).This work, published in Advanced Functional Materials under the title “Breast Cancer Bone Metastasis Therapy and Tumor-Associated Bone Destruction Repair by Versatile Semiconducting Nanointegrators with X-Ray Adjuvant.”, presents a comprehensive strategy for simultaneous treatment of bone metastases and repair of osseous defects.
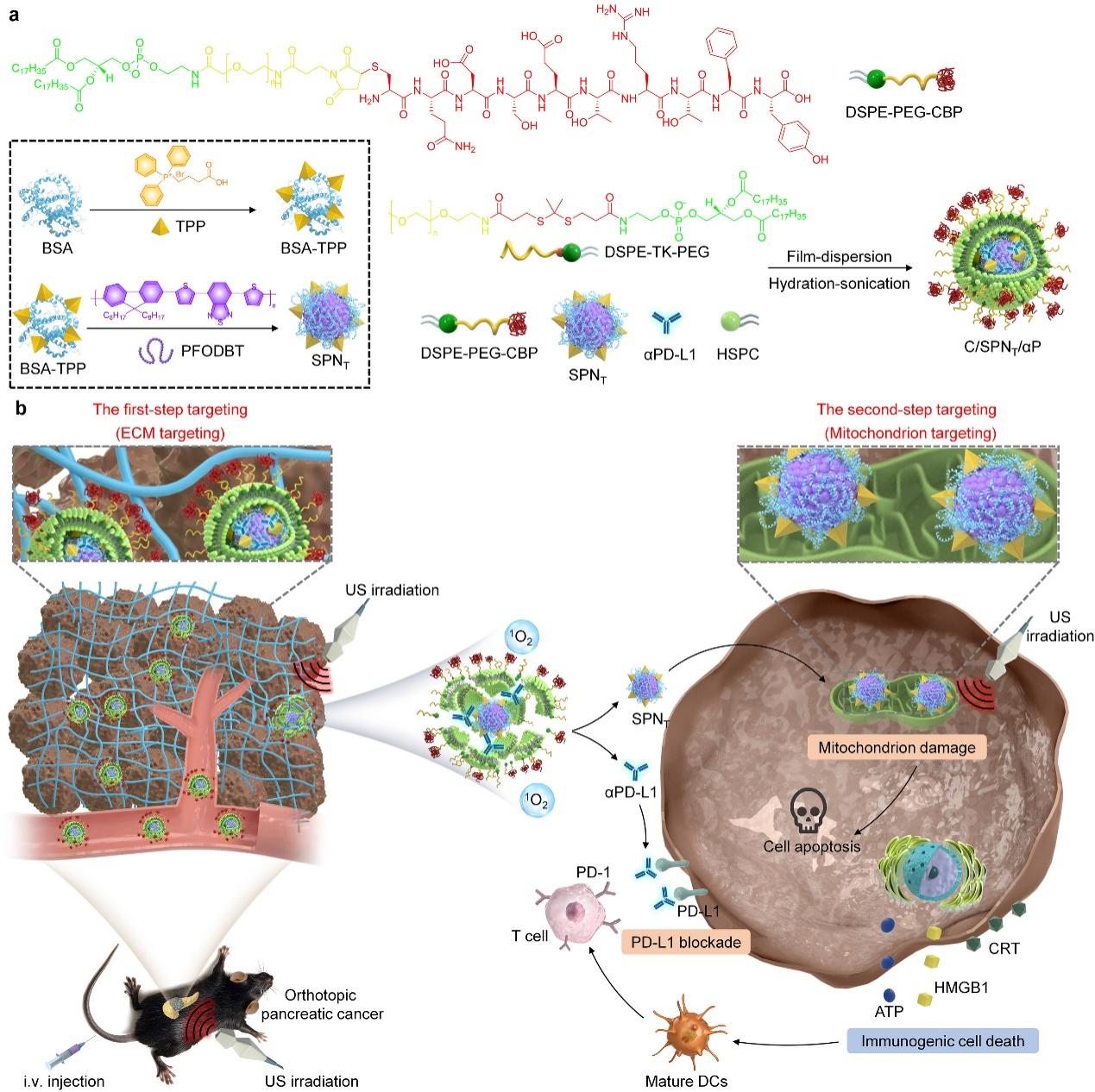
(Figure 2. Schematic diagram of the synthesis route of the two-step targeting-tunable C/SPNT/αP and its application for orthotopic pancreatic cancer therapy)
For orthotopic pancreatic cancer, the team constructed a semiconducting nanoswitch (C/SPNT/αP) capable of amplifying mitochondrial damage and PD-L1 blockade. This nanosystem embeds small nanoparticles (SPNT) containing a mitochondrial-targeting ligand (TPP) and semiconducting polymer (SP), together with αPD-L1, into ultrasound-responsive nanoliposomes conjugated with a collagen-binding peptide (CBP) for extracellular matrix targeting. In the first step, CBP facilitates accumulation of C/SPNT/αP at the pancreatic tumor site. Under ultrasound irradiation, the shell ruptures through sonodynamic therapy (SDT), releasing SPNT and αPD-L1. Subsequently, SPNT achieves mitochondrial targeting and enhances apoptosis via ^1O2 generation, thereby promoting immunogenic cell death (ICD). This immunostimulatory effect is further amplified by αPD-L1, which blocks the PD-L1 immune checkpoint (Fig. 2). This combined sonodynamic and immunotherapy approach exhibited strong antitumor efficacy in Panc02 and KPC orthotopic pancreatic cancer models, achieving effective tumor suppression by enhancing both ICD and T-cell activation. These findings were published in Advanced Functional Materials under the title “Two-Step Targeting-Tunable Semiconducting Nanoswitches Amplify Mitochondrion Damage and PD-L1 Blockade for Orthotopic Pancreatic Cancer Therapy.”
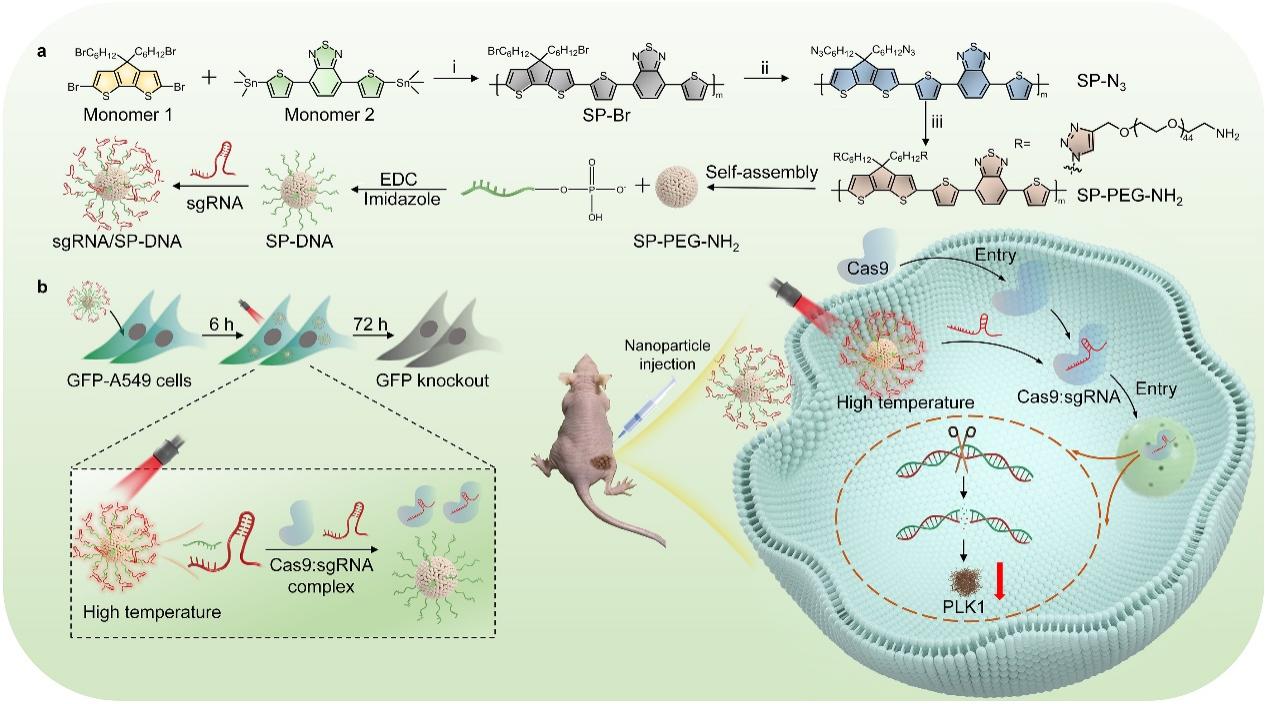
(Figure 3. Schematic diagram of NIR-photoactivatable organic polymeric nanocarrier for CRISPR activity regulation and tumor gene therapy)
To address the clinical challenge of achieving specific enrichment of CRISPR systems at lesion sites while minimizing off-target effects, the team developed a near-infrared (NIR) light-responsive organic polymeric nanocarrier for CRISPR activity regulation and tumor gene therapy. By grafting single-stranded DNA (ssDNA) complementary to the target region of the sgRNA onto a semiconducting polymer (SP) with strong photothermal conversion performance, they created an ssDNA-modified carrier (SP-DNA). The ssDNA on SP-DNA hybridizes with sgRNA to form a nano-system, which functions as both a delivery vehicle and an NIR photothermal conversion medium. Under NIR irradiation, localized heating causes the dissociation of sgRNA from ssDNA, releasing free active sgRNA and enabling precise regulation of CRISPR/Cas9 activity (Fig. 3). This strategy significantly reduces off-target effects while maintaining high gene editing efficiency, as demonstrated in tumor models. The research was recently published in Nano Letters under the title “A Semiconducting Polymer NanoCRISPR for Near-Infrared Photoactivatable Gene Editing and Cancer Gene Therapy.”


