Peripheral nerve injury often results in sensory and motor dysfunction, seriously impairing patients’ quality of life. Although existing nerve conduits can provide physical bridging, their limited topological structure, inadequate mechanical properties, and lack of electrical signal transmission capability hinder effective repair. Thus, developing nerve conduits that combine biomimetic architecture, suitable mechanical reinforcement, and electroactivity is crucial for restoring the nerve microenvironment.
Recently, Professor Huang Chen’s group from the School of Textile Science and Engineering at Donghua University, in collaboration with Chief Physician Ouyang Yuanming’s team at the Sixth People’s Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, fabricated a multifunctional nerve conduit by co-loading single-layer graphene (SLG) and nanodiamond (ND) particles into grooved polycaprolactone (PCL) fibers. The grooves guide oriented neurite extension, ND enhances mechanical strength, and SLG provides high electrical conductivity, synergistically rebuilding the nerve-regenerative microenvironment. This work, entitled “Anisotropic Single-layer Graphene/Nanodiamond-Loaded PCL Conduits Provide Biophysical Cues to Manipulate Nerve Biomechanics and Bioelectric Function in the Restoration of Nerve Microenvironment”, has been published in Advanced Functional Materials. Dr. Zhan Lei (Ph.D. candidate at DHU), Dr. Wang Xu, and Master’s student Lü Yaowei (Sixth People’s Hospital) are co-first authors of the paper.
Inspired by the anisotropic arrangement of natural nerve fascicles and the mechano-electrical properties of neural cells, SLG/ND/PCL microfibers were fabricated via electrospinning. Using polyvinylpyrrolidone (PVP) as a sacrificial template and applying solvent-induced phase separation, up to 14 aligned nano-grooves were created on each fiber surface. The specific process and principle are as follows:
PCL was dissolved using a combination of high- and low-volatility solvents. Groove formation during the electrospinning process proceeds through three distinct stages: (1) exchange between the high-volatility solvent and water vapor; (2) evaporation of water droplets, which generates pores, followed by stretching of semi-cured PCL fibers; and (3) complete curing of the fibers, resulting in groove formation. In this process, the introduction of PVP as a sacrificial template further increases both the number and depth of grooves on the fully cured PCL fibers. Compared with smooth fibers, these grooved structures provide aligned geometries and enlarged specific surface areas, which collectively promote enhanced cell adhesion and migration.
A custom-built device was employed to prepare double-layered conduits. The inner layer, composed of dual-oriented fibers (grooved plus aligned), directed nerve growth, while the outer layer of randomly oriented fibers enhanced mechanical strength. Incorporation of ND increased the compressive modulus by 1.6-fold due to strong ND–PCL interactions and the high intrinsic modulus of ND, while SLG endowed the conduits with an electrical conductivity of 0.012 S cm⁻¹ (Figure 1).
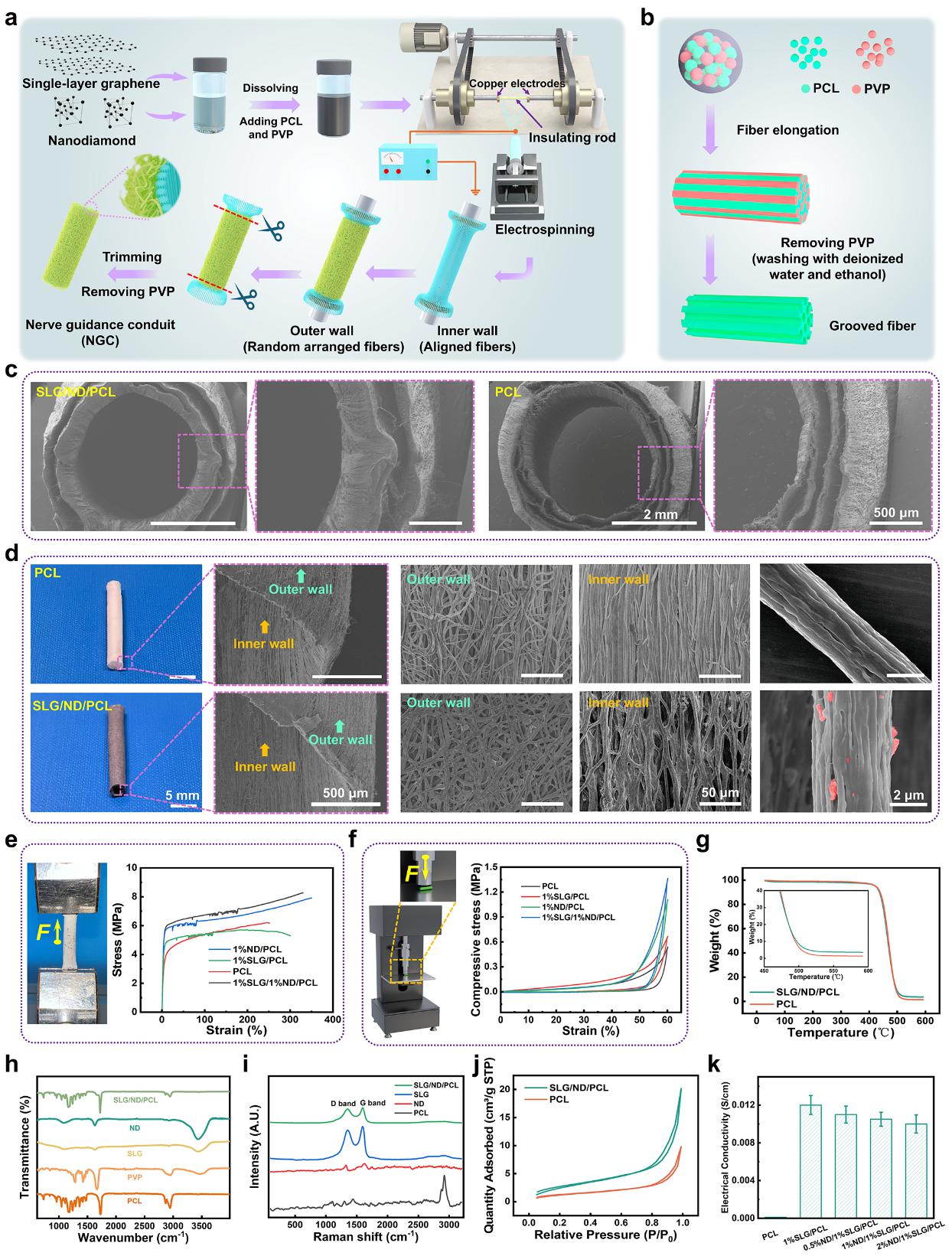
Figure 1. Fabrication process and performance characterization of the nerve conduit
Histological assessments of regenerated nerves in different groups were performed using HE staining, toluidine blue (TB) staining, Sirius red (SR) staining, and transmission electron microscopy (TEM). Overall, the SLG/ND/PCL group exhibited milder muscle atrophy, with axonal number, cross-sectional area, diameter, and myelin sheath thickness comparable to those of the autograft group and significantly greater than those observed in the control PCL group (Fig. 2).
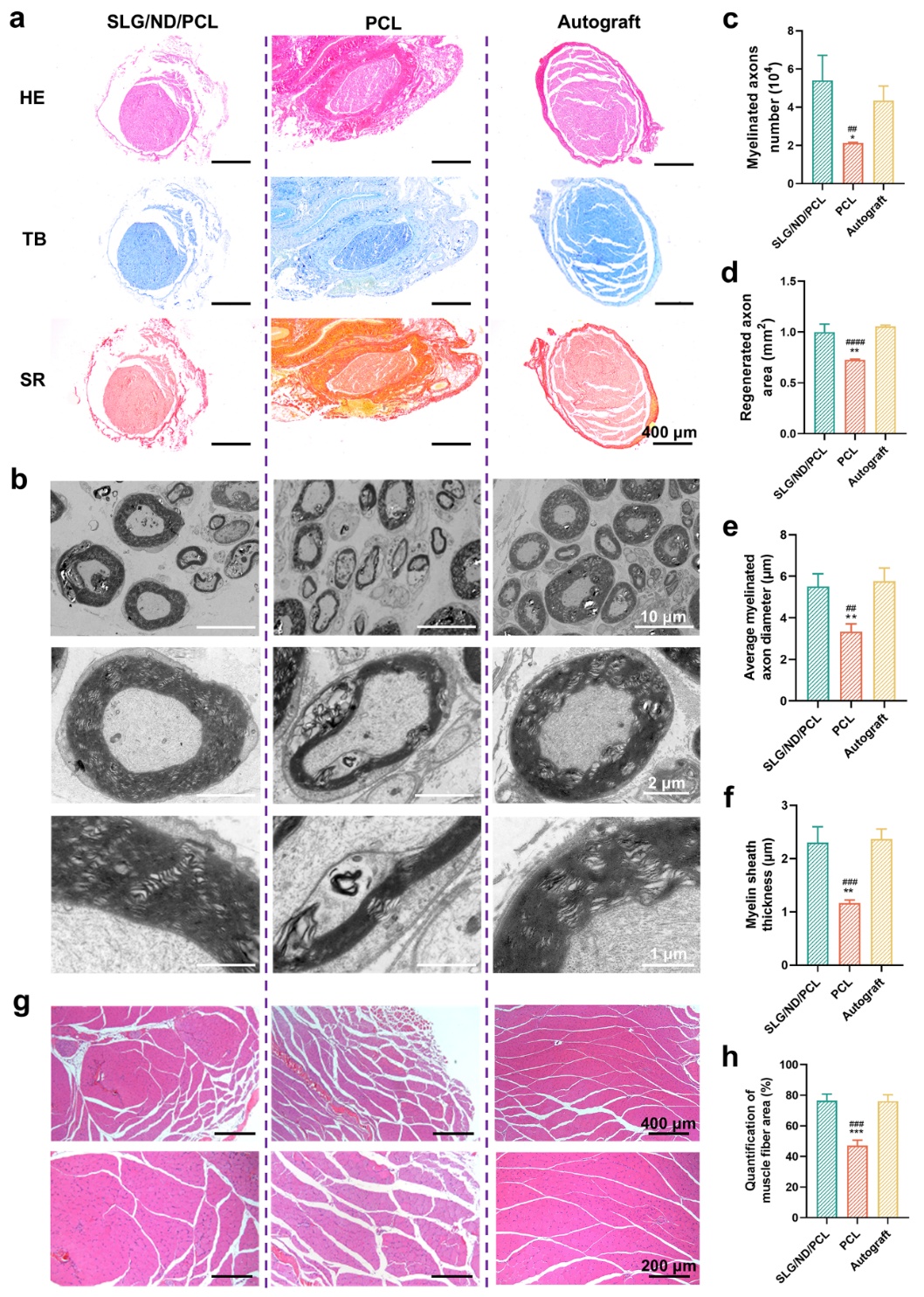
Figure 2. Expression of nerve restoration-related proteins promoted by the nerve conduits
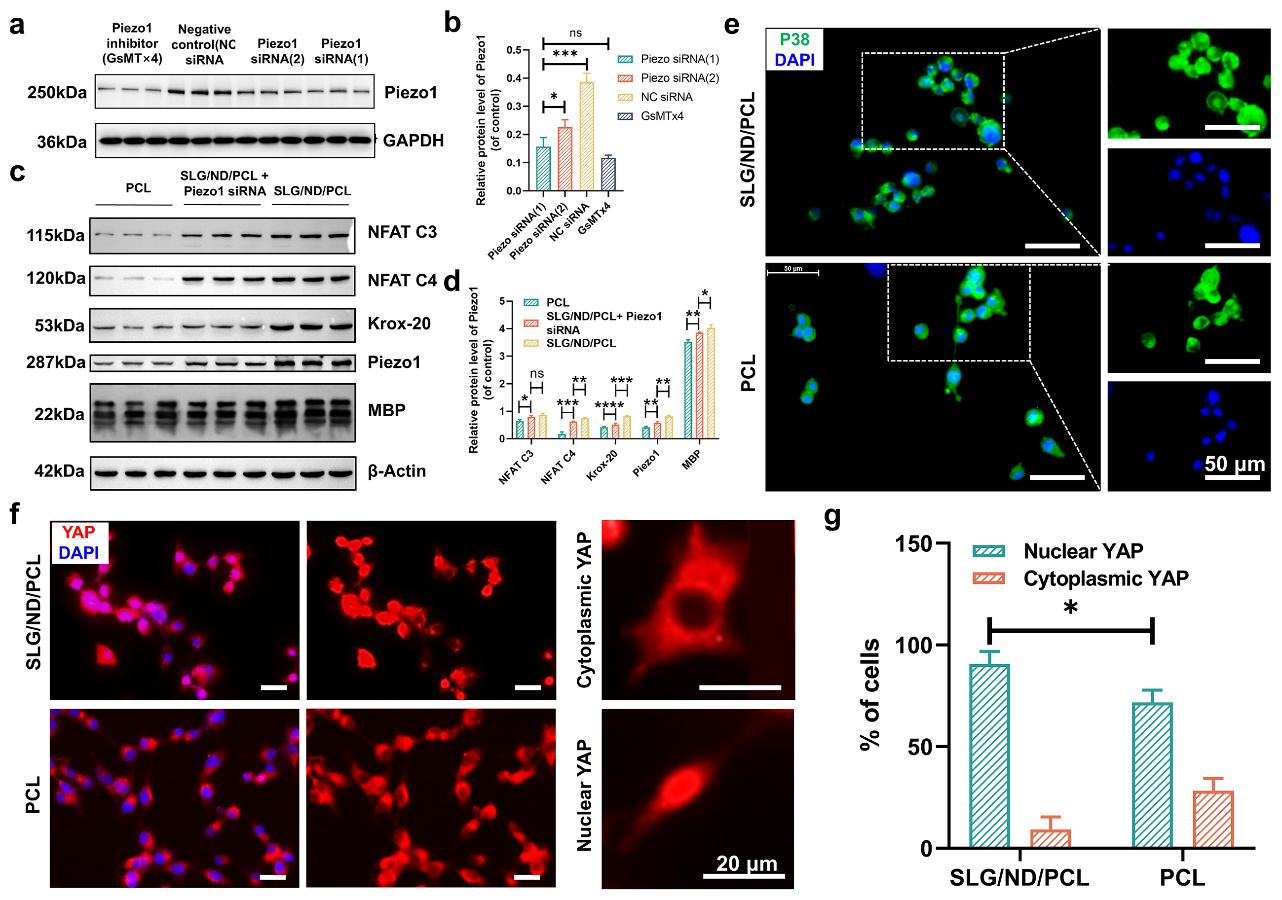
In the SLG/ND/PCL group, Schwann cells exhibited significantly enhanced expression of myelin basic protein. Moreover, following the knockout of Piezo1 gene expression, all proteins associated with myelination were downregulated, further confirming that the SLG/ND/PCL conduit promotes Piezo1 expression and activates related pathways, thereby facilitating NFAT signaling and subsequent myelination in a Piezo1-dependent manner (Fig. 3).
Figure 3. Mechanical deformation promotes restoration through the Piezo1 rather than the p38 signaling pathway
In summary, grooved PCL fibers co-loaded with SLG and ND were successfully fabricated into functional nerve conduits with enhanced mechanical reinforcement and electroactivity. By modulating Schwann cell growth and myelination in a Piezo1-dependent manner, these conduits substantially promoted peripheral nerve restoration.
This research was supported by the National Natural Science Foundation of China, the Shanghai Natural Science Foundation, the Fundamental Research Funds for the Central Universities, and the State Key Laboratory of Advanced Fiber Materials.