Recently, three representative research achievements in the field of nanomedicine by the team led by Professor Shi Xiangyang from the College of Biomedical Engineering were published in Advanced Functional Materials. These works propose innovative strategies for tumor therapy, including carrier-free full-active nanomedicines synthesized via microfluidics, dual-driven nanomotor metal-phenolic capsules, and dendrimer complex-mediated enzyme delivery systems, offering new solutions for precision cancer treatment.
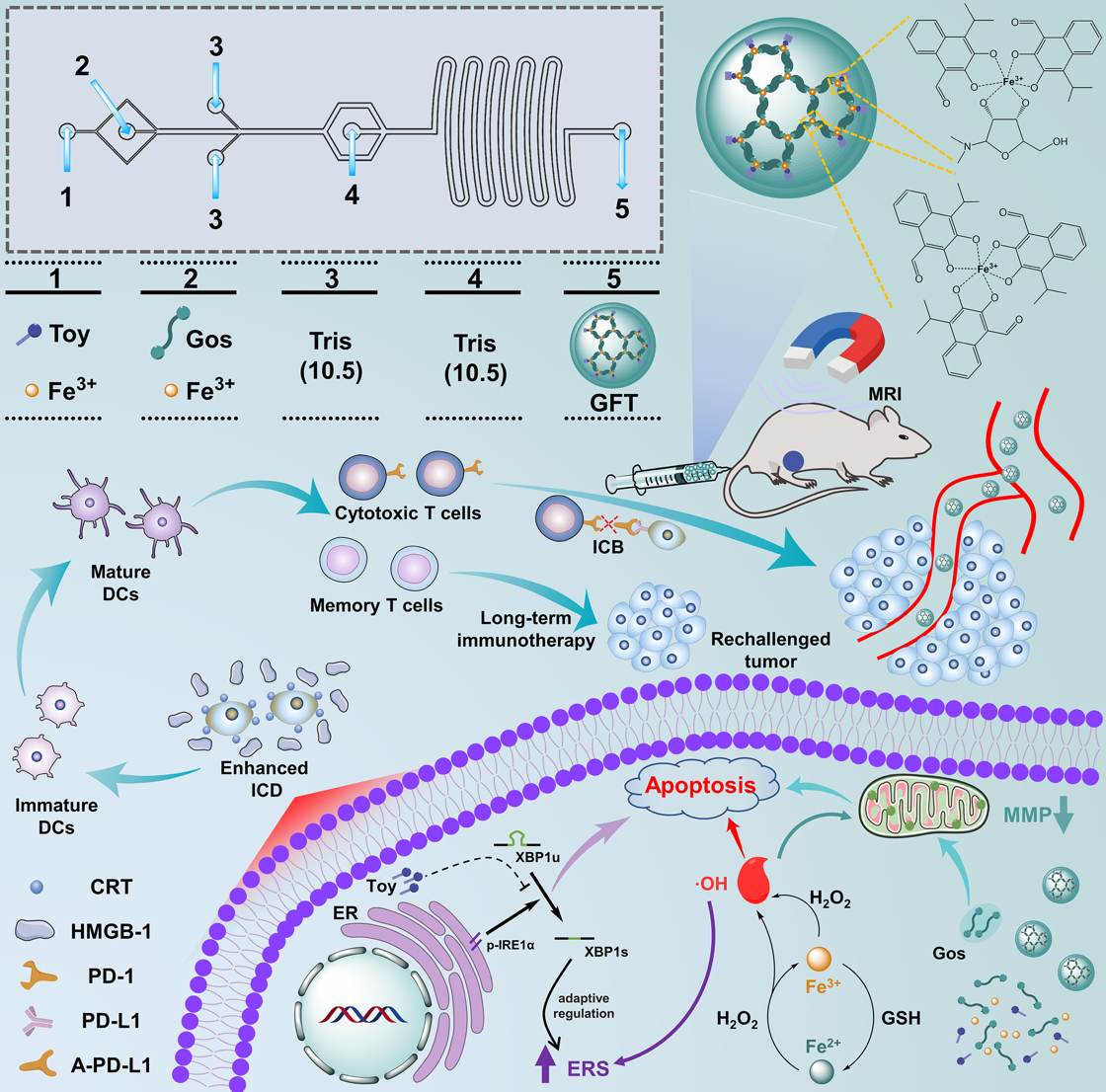
Figure 1. Schematic illustration of microfluidic synthesis of GFT NCs and their application in tumor theranostics
To construct efficient carrier-free nanoplatforms, the team developed self-assembled carrier-free gossypol–iron ion–toyocamycin nanocapsules (GFT NCs) using a one-step microfluidic method for tumor magnetic resonance (MR) imaging and chemo-chemodynamic-immune therapy (Figure 1). The nanocapsules possess favorable pH- and ROS-responsive drug release profiles and can be precisely delivered to tumor sites under MR imaging guidance. Through the synergistic effects of gossypol-induced mitochondrial dysfunction, Fe³⁺-mediated chemodynamic therapy, and toyocamycin-amplified endoplasmic reticulum stress, the GFT NCs effectively suppress tumor growth and metastasis while triggering potent antitumor immunity. Combined with programmed cell death ligand 1 (PD-L1) antibodies, they further boost immune activation and generate tumor-specific immune memory, preventing recurrence. The findings were published under the title “Microfluidic Synthesis of Carrier-Free Full-Active Metal-Phenolic Nanocapsules for Tumor Chemo-Chemodynamic-Immune Therapy.”
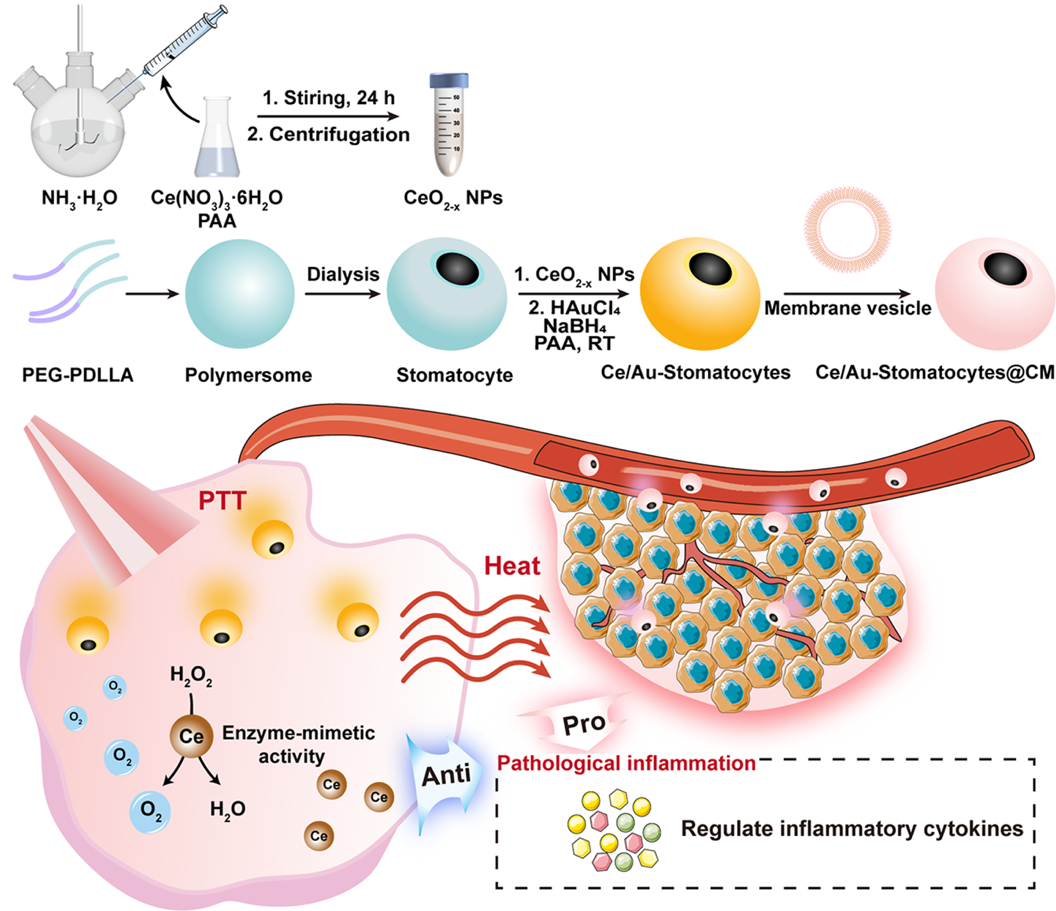
Figure 2. Schematic illustration of the synthesis of Ce/Au-Stomatocytes@CM and its application in in vivo antitumor photothermal therapy and inflammation alleviation
To enhance the efficacy of photothermal therapy (PTT) and mitigate post-treatment inflammation, the research team designed a dual-driven nanomotor based on bowl-shaped polymer vesicles (stomatocytes). Within this system, cerium oxide nanoparticles (CeO₂₋ₓ NPs), known for their multi-enzyme-mimetic activities and anti-inflammatory properties, were incorporated. Using a straightforward preparation method, the team developed a nanomedicine surface-modified with gold nanoparticles (Au NPs), internally loaded with CeO₂₋ₓ NPs, and bionically camouflaged with cancer cell membranes (CM) on the outer surface. This multifunctional nanomedicine acts as a dual-driven nanomotor for motion-enhanced tumor PTT (Figure 2). The nanomotor exhibits homotypic targeting, allowing it to accumulate selectively at tumor sites. Under laser irradiation, it can autonomously propel itself to penetrate tumor tissues, thereby improving photothermal treatment efficiency. Simultaneously, the CeO₂₋ₓ NPs generate oxygen within the hypoxic tumor microenvironment through enzyme-mimetic reactions. This oxygen not only fuels the nanomotor’s motion but also alleviates tumor hypoxia, further enhancing PTT outcomes. In addition, CeO₂₋ₓ NPs scavenge reactive oxygen species, helping regulate inflammatory responses and reduce skin damage after therapy. The study, entitled “Oxygen and Heat Dual-Driven Stomatocyte Nanomotors for Highly Efficient Inflammation-Relieved Breast Tumor Photothermal Therapy,” has been published online.
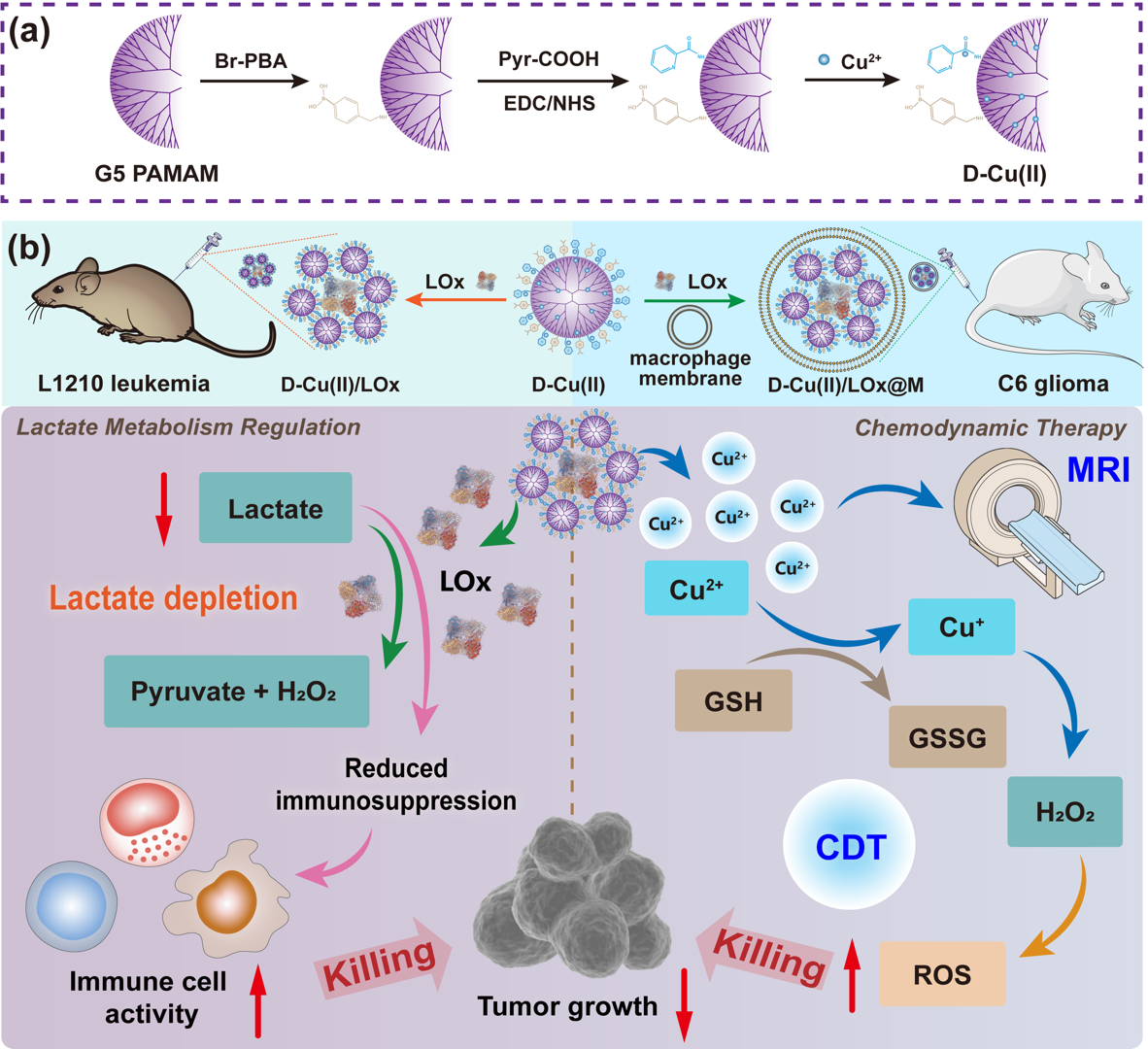
Figure 3. (a) Synthesis of D-Cu(II) complex and (b) mechanism of action in the treatment of L1210 leukemia and C6 glioma
To reduce lactate accumulation in the tumor microenvironment (TME) and thereby enhance antitumor immune responses, the research team developed a dendrimer–Cu(II) complex-mediated lactate oxidase (LOx) delivery system (D-Cu(II)/LOx) for lactate depletion-enhanced combinational therapy of glioma and leukemia. The system employs polyamidoamine (PAMAM) dendrimers as carriers, surface-modified with phenylboronic acid (PBA) and pyridine (Pyr), and coordinated with Cu(II) for efficient LOx delivery (Figure 3). The resulting dendrimer-based nanomedicine, D-Cu(II)/LOx, performs multiple therapeutic and diagnostic functions. It delivers LOx to disrupt lactate metabolism, while Cu(II) enables chemodynamic therapy and T1-weighted magnetic resonance imaging. Furthermore, in combination with the immune activator leukadherin-1 (LA-1), it remodels the immunosuppressive TME, significantly enhancing the therapeutic efficacy of LA-1 and strengthening antitumor immune responses. By camouflaging the system with macrophage membranes, the nanomedicine is also able to cross the blood–brain barrier, effectively treating orthotopic murine glioma models. This work provides a novel strategy that integrates metabolic intervention and chemodynamic therapy to achieve enhanced immunotherapy against tumors. The findings were published online under the title “Dendrimer-Cu(II) Complexes Mediate Enzyme Delivery for Lactate Depletion-Enhanced Combinational Treatment of Leukemia and Glioma.”
Multiple advances achieved by the university’s research teams in precision tumor therapy continue to offer innovative solutions for cancer treatment.


