Sodium-ion batteries (SIBs) have emerged as a promising next-generation secondary battery technology owing to the abundant reserves, wide distribution, and low cost of sodium resources. They hold great potential in the energy storage sector as a supplement to lithium-ion batteries, contributing to energy security. However, compared with lithium ions, sodium ions possess a larger ionic radius and higher atomic mass, with a mass-to-charge ratio (about 23) approximately 3.3 times that of lithium ions (about 6.94). These intrinsic properties result in slower ion transport and uneven flux, which in turn lead to low Coulombic efficiency (CE) and dendrite growth, severely compromising cycle life. Current strategies to improve ion transport near the cathode typically involve regulating the solvation structure to lower desolvation energy when ions transfer from liquid to solid phases. Yet, according to the principles of charge conservation and electrical neutrality, the total current in the electrolyte must equal the sum of charges carried by both cations and anions under the electric field. As a result, this traditional method of enhancing kinetics is reaching its theoretical limit.
To address the issue of cycle life reduction caused by unstable anode interfaces in SIBs, researchers from the State Key Laboratory of Advanced Fibrous Materials and the College of Materials Science and Engineering proposed regulating the composition of the solid electrolyte interphase (SEI) on the anode through separators with high dipole moments. Their related findings were published in Angewandte Chemie International Edition (2025, 64, e202415283).
More recently, the team introduced a strategy to optimize solvation structure by designing anionic anchoring separators (AAS). These separators can effectively trap free anions in the bulk phase, reduce their migration, and thereby enhance sodium-ion diffusion flux. Ion transport kinetics studies confirmed that AAS markedly decrease the number of anions reaching the cathode, weaken the anion solvation effect, and increase the proportion of solvent-separated ion pairs (SSIPs).
This work, entitled “Enhancing Robustness and Charge Transfer Kinetics of Sodium-ion Batteries through Introduction of Anionic Anchoring Separators”, was published in Journal of the American Chemical Society (2025, 147, 8488–8499). The paper lists master’s student Li Xiao as the first author, with Professors Zhu Meifang and Xu Guiyin as corresponding authors, and doctoral student Zhang Tao as co-author.
During discharge, free sodium ions and SSIPs carry positive charges and migrate toward the cathode, facilitating current transmission. In contrast, anion-containing aggregates (AGGs) migrate toward the anode, contributing negatively to current transmission and raising internal resistance (Figure 1a). To overcome this, the team carefully designed AAS to regulate bulk solvation structure, and analyzed Na⁺ solvation through stoichiometric probability density of solvated clusters. Results show that anionic anchoring effectively restricts anions and promotes SSIP formation, enabling SSIPs to act as primary charge carriers that improve charge transfer kinetics in SIBs.
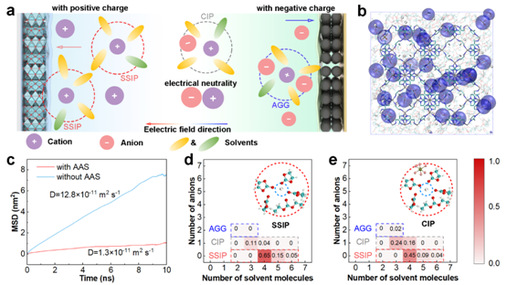
Figure 1: The influence of anionic anchoring and simulation of solvation structure
The researchers also established a nuclear magnetic resonance (NMR)-assisted Hittorf method to study ion transport under an electric field and quantify anion transfer kinetics (Figure 2d). The results verified that AAS effectively suppress PF₆⁻ migration. Although this method involves an unrealistic separator-to-electrolyte volume ratio, it suggests that the true anchoring effect of AAS may be even stronger. Complementary in-situ attenuated total reflection Fourier transform infrared spectroscopy (ATR-FTIR) further confirmed the impact of AAS on ion transport behavior.
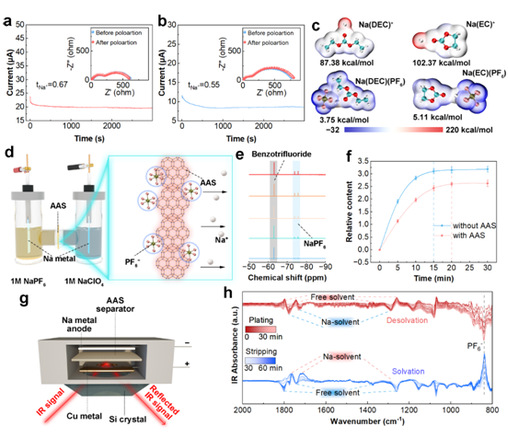
Figure 2: Study on the influence of AAS on Na⁺ Transfer Kinetics
Benefiting from enhanced Na⁺ transfer kinetics, the Na||Cu battery equipped with AAS achieved a longer Sand’s time (3.55 h vs. 1.25 h for GF) (Figure 3a). In Na||Na symmetric batteries, sodium deposition after 50 cycles appeared denser and more uniform (Figure 3b), while plating/stripping tests (Figures 3d, 3e) demonstrated excellent reversibility and lower overpotential. These results indicate that AAS can reduce internal resistance, improve plating/stripping stability, increase critical current density (CCD) and CE, and maintain reversibility even under harsh low-capacity-ratio conditions—thereby enhancing anode stability, reducing cell volume, and improving energy density.
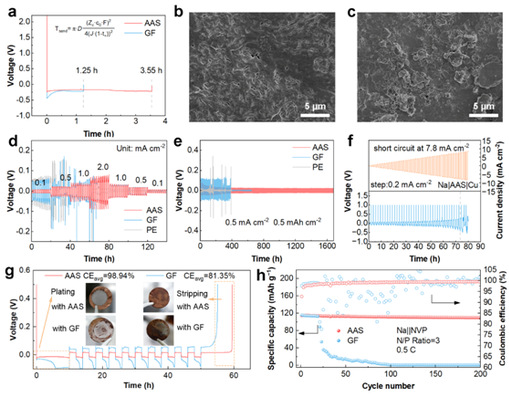
Figure 3: Characterization of anode stability and plating/stripping reversibility
Furthermore, anions are known to lower solvent oxidative stability, compromising cathode electrolyte interphase (CEI) integrity. Linear sweep voltammetry (LSV) showed that confining anions raises oxidation potential, mitigating parasitic reactions on the cathode side (Figure 4c). With AAS, a thinner and more uniform CEI was formed. At low electrolyte-to-cathode ratios, the Na|AAS|NVP battery exhibited superior cycling stability, as AAS alleviated cathode failure risks and reduced side reactions (Figure 4f). X-ray photoelectron spectroscopy (XPS) further revealed that CEI films in the GF system contained higher organic and lower inorganic content, making them thicker and more resistive than those formed in the AAS system (Figures 4i, 4j). This demonstrates that AAS improve solvent oxidative stability and enable formation of a stable CEI.
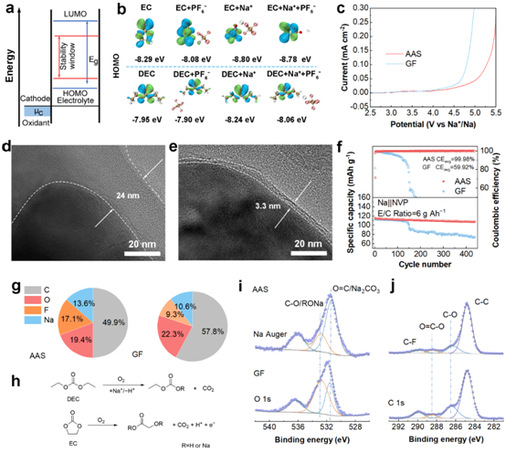
Figure 4: The influence of AAS on cathode stability
At 0.5 C, the HC|AAS|NVP full cell showed reduced capacity fading and retained 91.35% of its capacity with an average CE of 99.5% (Figure 5c). Meanwhile, the HC|AAS|NVP pouch cell achieved an impressive energy density of 225.7 Wh kg⁻¹ at 0.2 C (based on total cell mass), along with high capacity (176 mAh) and excellent cycling stability (Figure 5f). These results highlight that AAS allow SIBs to maintain cycling stability with lean electrolyte and high cathode loading, thereby reducing inactive mass and volume, lowering costs, and improving energy density.
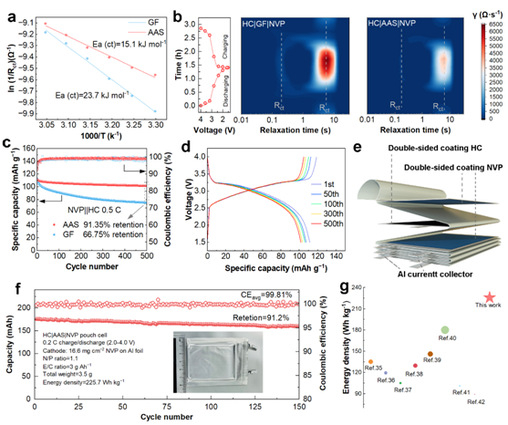
Figure 5: Performance of HC|AAS|NVP full cell
The anionic anchoring strategy proposed by the DHU research team provides a new direction for designing functional separators and opens promising opportunities for realizing next-generation safe, rechargeable sodium-ion batteries.


