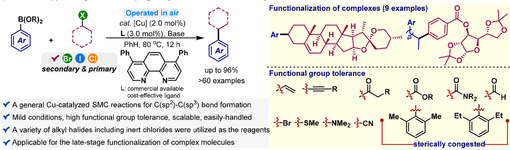
The Suzuki-Miyaura reaction has been proven to be highly effective in constructing C(sp2)-C(sp2) bonds. However, its performance in the transformation of alkyl halides with sp3 hybridization still leaves something to be desired. The development of a general C(sp2)-C(sp3) methodology would be highly significant and widely welcomed. Recently, the research group led by Professor Xie Weilong has developed a concise and efficient copper catalytic system that enables the coupling reaction between aryl boronic acids and non-activated primary and secondary alkyl halides. This system operates under relatively mild conditions and is compatible with various alkyl halides, including iodides, bromides, and chlorides. It has shown broad substrate compatibility with different functional groups and can accommodate sterically hindered substrates and complex molecules. Notably, the reaction can be conducted in air to avoid the need for commonly used inert environments, thus providing a practical coupling method.
The recent research achievement titled A General Copper Catalytic System for Suzuki-Miyaura Cross-Coupling of Unactivated Secondary and Primary Alkyl Halides with Arylborons has been published in the renowned international journal Journal of the American Chemical Society. Professor Xie Weilong from Donghua University is the corresponding author of the paper, and Zhou Yonglei, a master’s student from the College of Chemistry and Chemical Engineering, is the first author. And Donghua University is the sole corresponding institution for this publication.