In addressing the challengesin accurate diagnosis and efficient treatment of early-stage cancers, the research team led by Prof. Li Jingchao from DHU College of Biomedical Science and Medical Engineering has made remarkable strides. It has successfully engineered a suite of activatable nanomedicine delivery systems tailored for precise cancer diagnosis and treatment and yielded innovativeresearch outcomes.The team has published a series of articles in internationally renowned journals, includingAngewandte Chemie,Advanced Materials,NANO Letters, and NANO Today.
The team has devised near-infrared light and ultrasound-activated nanomedicine delivery systems that enable precise immune therapy for various tumors (published in NANO Today, 2023, 50, 101833). It has also developedsono-activatable semiconducting polymer nanopartners for immunotherapy, referred to as SPNTi (published in Advanced Materials, 2023, 35, 2302508). The first author of the latter publication is Master’s research student DingMengbin.The nanomedicine achieves site-specific drug delivery through ultrasonically triggered mechanisms. In comparison to conventional drug delivery nanosystems and those activated by the tumor microenvironment, this innovation ensures more precise drug delivery and immune therapy effects.
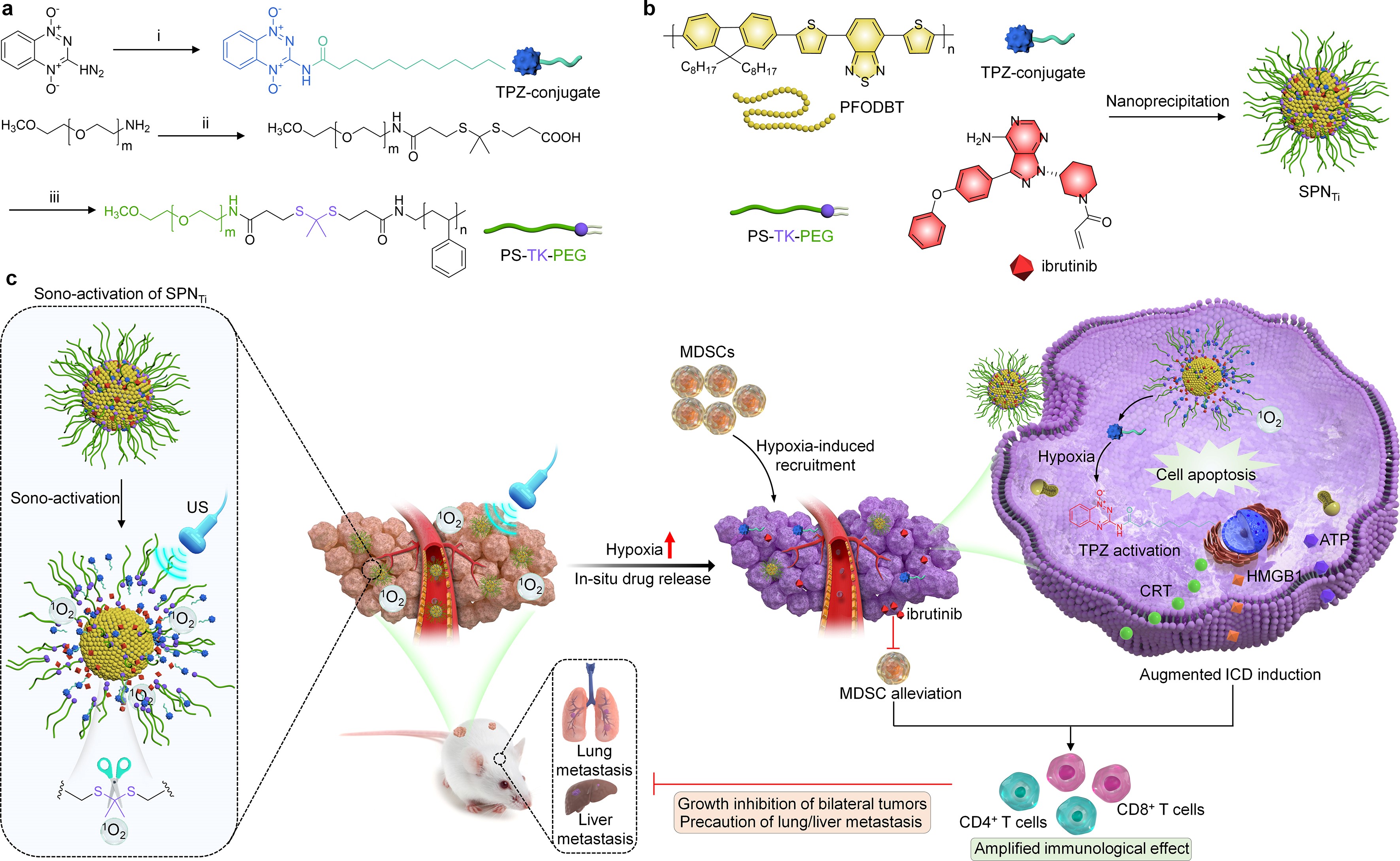
Additionally, the team collaborated with Prof. Pu Kanyi from Nanyang Technological University, Singapore and Assoc. Prof.Luo Yu from Shanghai University of Engineering Science to create semiconducting polymer pro-nanomodulators for deep-tissue sono-immunotherapy of orthotopic pancreatic cancer (published in Angewandte Chemie, 2023, 62, e202305200).Moreover, the team recently developed sono-activatable semiconducting polymer nanofeedbacks for dual-modulation of immunosuppressive pathways in cancer immunotherapy (published in NANO Today, 2023, 52, 101944). Furthermore, Prof, Li Jingchao designed activatable semiconducting polymer nanoinducers, which can amplify oxidative damage via sono-ferroptosis to achieve synergistic therapy of bone metastasis.


